Ester Definition
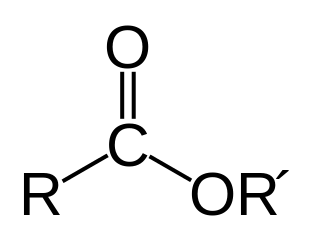
An ester is a group of organic compounds that are derived from organic acids where at least one –OH group is replaced by an -O- group.
- The functional group in ester is the ester group (COOR) which is usually formed as a result of a substitution reaction between carboxylic acids and organic alcohols.
- Esters are essential organic compounds that form the structural basis of essential biomolecules like lipids.
- The chemical formula of esters is RCOOR’, where the R and R’ can be the same or different organic groups.
- The process of formation of esters is called esterification, which is the reaction between carboxylic acids and alcohols in the presence of acid.
- Esters contain a carbonyl group which is then attached to an oxygen atom to form the functional group. Esters are named with the suffix ‘-ate’ by the IUPAC.
- Due to the presence of a carbonyl center, esters have 120° C-C-O and O-C-O angles, and the functional groups of these esters are flexible.
- The flexibility of the angles allows for certain physical properties of the compounds, like lower melting point and increased volatility.
- The presence of the carbonyl group also increases the polarity of the compound as the charge developed can be distributed to the carbon and oxygen to generate a stable structure.
- Esters are mostly used in food industries for the incorporation of the aroma of many fruits like apples, bananas, pears, etc.
- Esters also occur in fats in the form of trimesters which are essential for the structure and energy of living systems.
Ether Definition

Ether is a group of organic compounds consisting of the ether group -O- connecting two alkyl or aryl groups.
- The chemical formula of ether is R-O-R’.
- Ethers can be broadly classified as symmetrical and mixed or asymmetrical ethers depending on the type of alkyl or aryl groups present on the sides of the oxygen atom.
- If the same alkyl or aryl groups are present, it is a simple or symmetrical ether, but if the groups are different, the ethers are called mixed or asymmetrical ethers.
- The C-O linkage in ethers is bent, usually at an angle of 111°, and the bond is flexible for rotation.
- Ethers are more acidic than simple hydrocarbons as well as esters, as the oxygen atom is more electronegative than carbon.
- Based on the IUPAC nomenclature system, ethers are named by the general formula, ‘alkoxyalkane’.
- Ethers usually have a lower boiling point and are less soluble than the respective alcohols. These occur in the form of pleasant-smelling colorless liquid.
- Different ethers have different applications in various industrial fields as some are essential in medicine and pharmacology for the preparation of anesthetics.
- Ethyl ether, in particular, is used as an excellent solvent for the extraction of various chemicals.
- The ability of ethers to be used as solvents due to the possibility of the formation of hydrogen bonds with other molecules.
- Ethers can be prepared artificially by the dehydration of alcohols and alkyl halide. The most popular method of synthesis of ethers is via the Williamson ether synthesis.
12 Key Differences (Ester vs Ether)
| Characteristics | Ester | Ether |
| Definition | An ester is a group of organic compounds that are derived from organic acids where at least one –OH group is replaced by an -O- group. | Ether is a group of organic compounds consisting of the ether group -O- connecting two alkyl or aryl groups. |
| Chemical formula | The chemical formula of the ester is RCOOR’. | The chemical formula of ether is R-O-R’. |
| Functional group | The functional group in ester is –COO- which is also called the ester group. | The functional group in ether is –O- which is also called the alkoxy group. |
| Carbonyl group | Esters contain a carbonyl group. | No carbonyl group is present in ethers. |
| Nomenclature | Esters are provided with the suffix ‘-ate’ according to IUPAC rules. | Ethers are named alkoxyalkanes. |
| Derived from | Esters are derived from carboxylic acids. | Ethers are derived from alcohol. |
| Odor | Esters have a fruity smell. | Ethers have a strong alcoholic smell. |
| Polarity | Esters are more polar than ethers due to the presence of the carbonyl group. | Ethers are less polar than esters. |
| Boiling Point | The boiling point of esters is lesser than the carboxylic acids and alcohols of the same weight. | The boiling point of ethers is even lower than the esters, carboxylic acids, and alcohols of the same weight. |
| Symmetrical structures | Esters do not have symmetrical structures due to the presence of a carbonyl group. | Ethers can be symmetrical if the alkyl and aryl groups bonded to the oxygen atom are similar. |
| Uses | Esters are commonly used in the food industry to enhance the taste and odor of the product. | Ethers are commonly used in drugs as solvents. |
| Examples | Some examples of esters are methyl acetate, ethyl propionate, etc. | Some examples of ethers are methoxy ethane, phenoxy benzene, etc. |
Examples of Esters
Ethyl acetate
- Ethyl acetate or ethyl ethanoate is an ester with the molecular formula CH3-COO-CH2-CH3.
- Ethyl acetate is synthesized from acetic acid and ethanol by the process of esterification in the presence of an acid.
- It is primarily used as a solvent because of its low cost, low toxicity, and fruity odor. It is used as a solvent for the preparation of medicines and other pharmaceutical products.
- Ethyl acetate also occurs in wine as a product of volatile organic acid and ethyl alcohol produced during fermentation.
- Besides, it also has applications in laboratories for column chromatography and extractions of different chemicals.
- Ethyl acetate is also useful in entomology as an asphyxiant during insect collection and studies.
Examples of Ethers
Diethyl ether
- Diethyl ether is an organic compound composed of two carbon atoms with the molecular formula (C2H5)2O.
- It exists as a clear, colorless liquid with an anesthetic odor. It is less dense than water and soluble in water.
- Diethyl ether is symmetrical ether as the oxygen atom is bonded to two ethyl groups.
- Diethyl ether is produced by the hydration of ethylene both in laboratories as well as industries.
- It is a highly flammable liquid that is used for combustion to produce carbon dioxide and water.
- It has a high cetane number and is used as a starting fluid for the production of petroleum distillates and gasoline.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 3283, Ether” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ether. Accessed 18 March 2021.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 8857, Ethyl acetate” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-acetate. Accessed 18 March, 2021.
- 2% – https://www.scribd.com/presentation/360055436/Mineral-Compounds-Autosaved
- 2% – https://www.coursehero.com/file/12238622/prelab-6/
- 1% – https://www.scienceabc.com/pure-sciences/what-are-esters-formation-properties-and-uses.html
- 1% – https://www.chemguide.co.uk/organicprops/alcohols/esterification.html
- 1% – https://www.britannica.com/science/ester-chemical-compound
- 1% – https://kylefoster.me/acetato-de-etilo-msds-67/
- 1% – https://en.wikipedia.org/wiki/Ether
- 1% – https://en.wikipedia.org/wiki/Diethyl_ether
- 1% – https://en.unionpedia.org/Kilogram
- 1% – https://courses.lumenlearning.com/trident-boundless-chemistry/chapter/functional-group-names-properties-and-reactions/
- 1% – https://courses.lumenlearning.com/introchem/chapter/esters/
- 1% – https://byjus.com/chemistry/difference-between-ester-and-ether/
- 1% – http://www.3rd1000.com/chem301/chem301w.htm
- <1% – https://www.britannica.com/science/ether-chemical-compound
- <1% – https://quizlet.com/gb/369592735/carbonyl-compounds-flash-cards/
- <1% – https://pubchem.ncbi.nlm.nih.gov/compound/Dimethyl-ether
- <1% – https://en.wikipedia.org/wiki/Butyl_acetate
- <1% – https://courses.lumenlearning.com/boundless-chemistry/chapter/aromatic-hydrocarbons/
- <1% – https://chemistryrack.com/what-are-esters/
- <1% – https://byjus.com/chemistry/ether-preparation/
- <1% – https://byjus.com/chemistry/diethyl-ether/
