An ionic solid dissolves in water, which causes the crystal lattice to disintegrate and the ions to disperse. Overcoming the attractive forces between ions necessitates a significant amount of energy.
Enthalpy of Solution
The enthalpy of solution refers to the amount of heat released or absorbed when a solute dissolves in a solvent to form a solution.
The thermodynamic quantity that characterizes the enthalpy change occurring when a substance is dissolved in a solvent under constant pressure, leading to infinite dilution, is referred to as the enthalpy of solution, enthalpy of dissolution, or heat of solution. The unit commonly utilized for expressing solution enthalpy at a consistent temperature is kJ/mol.
- The energy change associated with the dissolution process comprises three fundamental constituents, namely, the endothermic dissociation of bonds existing between the solute and the solvent, and the concomitant establishment of attractive forces between the solute and the solvent.
- In a theoretical solution that is considered perfect, the enthalpy of mixing is determined to be zero. Non-ideal solutions are characterized by the presence of additional molecular weight.
- The phenomenon of exothermic dissolution is a prevalent occurrence in most gases. In other words, the exothermic reaction that occurs when a gas dissolves in a liquid solvent results in an increase in temperature of the solution and its environment.
- The temperature of the solution gradually decreases in order to equilibrate with the ambient surroundings. Le Châtelier’s principle predicts that the gas-solution equilibrium will favor the gas in solution as the temperature decreases. This is due to the fact that a decrease in temperature leads to an increase in the solubility of a gas.
What is enthalpy change of solution?
The enthalpy change of solution, ΔHosol, is the energy absorbed or released when 1 mole of an ionic solid dissolves in sufficient water to form a very dilute solution.
The enthalpy changes of solution for magnesium chloride and sodium chloride are described by the equations below:
- MgCl2(s) + aq → Mg2+ (aq) + 2Cl– (aq) ΔHosol = –55 kJ mol–1
- NaCl(s) + aq → Na+(aq) + Cl–(aq) ΔHosol= +3.9kJ mol–1
Here,
- ΔHosol: the symbol for enthalpy change of solution
‘aq’: the symbol represents the very large amount of water used - enthalpy changes of solution can be positive (endothermic) or negative (exothermic)
- a compound is likely to be soluble in water only if ΔHosol is negative or has a small positive value;
- substances with large positive values of ΔHosol are relatively insoluble
Enthalpy change of hydration
The enthalpy change of hydration, ΔHo hyd, is the enthalpy change when 1 mole of a specified gaseous ion dissolves in sufficient water to form a very dilute solution.
The lattice energy for sodium chloride is –788 kJmol–1. This means that we need to supply (at least) +788 kJmol–1 to overcome the forces of attraction between the ions. But ΔHosol [NaCl] is only +3.9 kJmol–1. The energy needed to separate the ions come from the strong attraction between the ions and the water molecules.
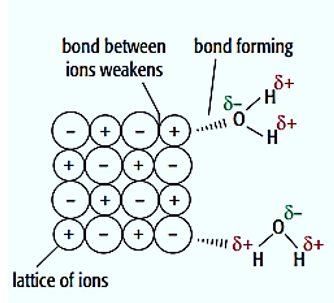
Upon dissolution of an ionic solid in water, the ions establish bonds with water molecules. The aforementioned chemical bonds are commonly referred to as ion-dipole bonds in academic literature. The molecule of water exhibits polarity
. The oxygen atoms with δ- charge in water molecules exhibit an attractive force towards the positively charged ions present in the ionic compound. The positively charged hydrogen atoms (δ+) present in water molecules exhibit an attractive force towards the negatively charged ions in the ionic compound.
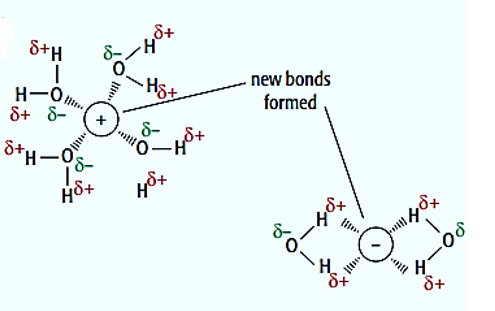
The energy that is required to separate the anions and cations that are bonded together in the crystal lattice is compensated by the energy released during the formation of ion-dipole bonds. The enthalpy change of hydration refers to the energy that is released when gaseous ions dissolve in water.
Example of Enthalpy change for hydration
The enthalpy changes of hydration for calcium ions and chloride ions are described by the equations below:
- Ca2+(g) + aq → Ca2+(aq) ΔHo hyd= –1650 kJmol–1
- Cl–(g) + aq → Cl– (aq) ΔHo hyd= –364 kJmol–1
Here;
- ΔHo hyd: the symbol for enthalpy change of hydration
- the enthalpy change of hydration is always exothermic
- the value of ΔHo hyd is more exothermic for ions with the same charge but smaller ionic radii, e.g. ΔHo hyd is more exothermic for Li+ than for Na+
- the value of ΔHo hyd is more exothermic for ions with the same radii but a larger charge, e.g. ΔHo hyd is more exothermic for Mg2+ than for Li+.
Calculating enthalpy changes in solution
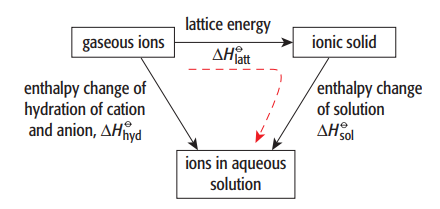
One can determine the enthalpy change of solution or hydration by creating an enthalpy cycle and applying Hess’s law.
enthalpy change of hydration and enthalpy change of solution.
From this enthalpy cycle;
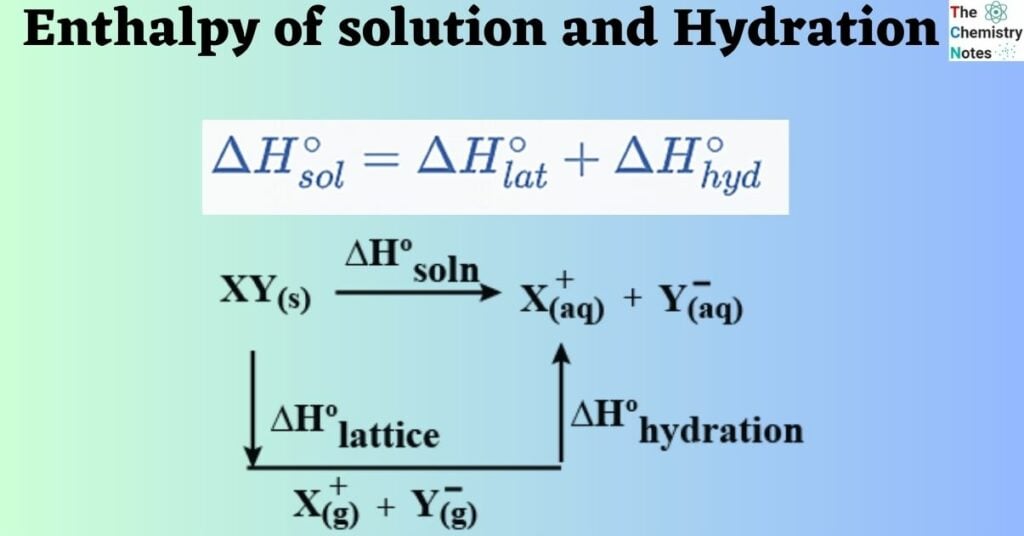
ΔHolatt + ΔHosol = ΔHohyd
Note that the ΔHohyd values for both anions and cations are added together to get the total value,
We can use this energy cycle to calculate:
- the value of ΔHosol
- the value of ΔHohyd
Hydration Enthalpy and Solubility
The cohesive force between ions in a solute is attributed to the Coulombic attraction. In order to achieve dissolution of the solute within the solvent, which in this case is water, it is necessary for the water molecule to overcome a formidable force of attraction. The thermodynamic quantity that represents the energy needed to overcome the force of attraction between the constituent particles of a solid and completely separate them into gaseous ions is referred to as lattice enthalpy.
The majority of ionic compounds exhibit limited solubility in non-aqueous solvents, yet they demonstrate significant solubility in aqueous solutions. The solubility of a salt is determined by the interaction between its constituent ions and the solvent. As previously elucidated, water is a polar molecule that exhibits a partial positive charge on its hydrogen atoms and a partial negative charge on its oxygen atom. This molecular polarity facilitates its interaction with ions, resulting in the formation of robust chemical bonds and the subsequent release of energy.
Change in hydration enthalpy down the group
- It has been observed that ions of smaller size exhibit greater enthalpy changes of hydration, despite having the same charge.
- As a result, the order of enthalpy change of hydration, from most to least exothermic, is Mg2+ > Ca2+ > Sr2+ > Ba2+
- The reduction observed down the group is considered significant and is solely attributed to the expansion in the cation’s size, while the anion remains constant, specifically the sulfate ion in all instances.
Change in lattice energy down the group
- If the ions forming the lattice have the same charge, the lattice energy will be greater if the ions are small. Therefore, the order of decreasing lattice energy is determined by the size of the ions.
- The order of the cations from highest to lowest charge is Mg2+ > Ca2+ > Sr2+ > Ba2+
- Additionally, the lattice energy is inversely proportional to the sum of the anion and cation radii.
- Since the sulfate ion is larger than the group 2 cations, it contributes more to the change in lattice energy down the group.
- As a result, the decrease in lattice energy is relatively smaller down the group and is determined more by the size of the sulfate ion rather than the size of the cations.
Difference in enthalpy change of solution of Group 2 sulfates
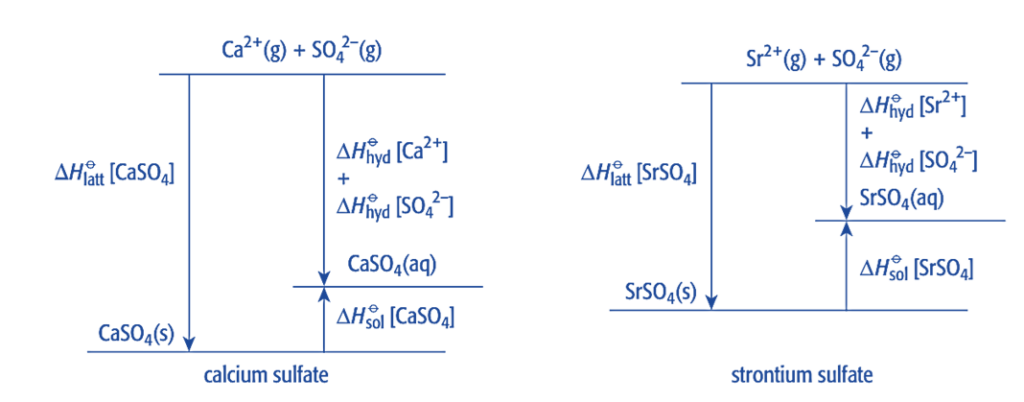
The lattice energy of sulfates decreases down the group, but by relatively smaller values, meaning it becomes less exothermic. Similarly, the enthalpy change of hydration decreases down the group, but by relatively larger values, making it less exothermic. By applying Hess’s law, we can conclude that the value of ΔHosol becomes more endothermic down the group. As a result, the solubility of Group 2 sulfates decreases down the group.
The relationship between Enthalpy of Solution and Hydration
- The enthalpy of solution refers to the amount of energy released when one mole of an ionic solution dissolves in water.
- The enthalpy of hydration refers to the amount of energy released when a single mole of gaseous ions is dissolved in water.
- The following equations can be used to calculate various thermodynamic concepts:
enthalpy of solution: XY (s) + aq → X (aq)+ + Y (aq)−
enthalpy of hydration: X (g)+ + aq → X (aq)+
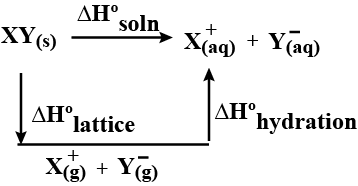
The relationship between these two concepts can be established through the lattice enthalpy, which is expressed by the following equation:

References
- https://alevelchemistry.co.uk/notes/enthalpy-changes/
- https://studymind.co.uk/notes/enthalpy-of-solution/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/Energies_and_Potentials/ Enthalpy/ Enthalpy_Change_of_Solution
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/enthalpy-of-solution-and-hydration/
- https://unacademy.com/content/neet-ug/study-material/chemistry/an-explanation-on-enthalpy-of-solution-with-examples/
- https://studymind.co.uk/notes/enthalpy-of-hydration/
- https://www.chemguide.co.uk/
- https://byjus.com/jee/hydration-enthalpy/
