Enolates have an essential role as organic reactive intermediates. They have the properties of both alkoxide and carbanion. Enolates may be made from carbonyl compounds like aldehydes and ketones by protonating the oxygen atom and then deprotonating the alpha-carbon atom. This is possible when an acid and a base are present. These intermediates can subsequently be employed in many reactions, such as the Aldol and Michael reactions. These are utilized in organic synthesis and are frequently employed in the creation of complicated compounds.
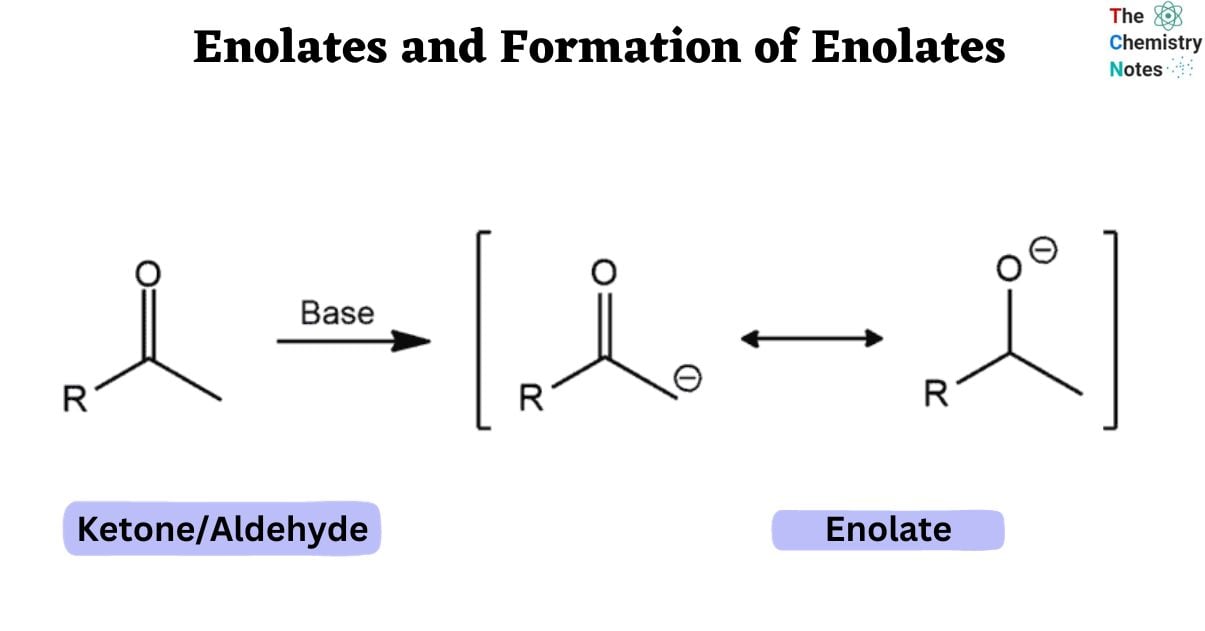
Because the stereochemical outcome of an enolate reaction is often influenced by the geometry of the enolate, enolate formation is a critical stage in many bond-forming processes.
Interesting Science Videos
Formation of Enolates
- Nucleophiles are enolates formed when a strong base abstracts the hydrogen atom. As bases, lithium diisopropylamide (LDA) or sodium hydride are required.
- The acidity of the two potential hydrogen atoms is related to the site of proton abstraction, which is in the order:
primary > secondary > tertiary- When an enolate interacts with an alkyl halide, alkylated ketones occur. Proton exchange between the original enolate and the alkylated ketone, followed by the alkylation of that enolate ion, may result in numerous alkylations.
- Enolates can also be synthesized using alkyl lithium reagents, enol esters, or silyl enol ethers. House found a mechanism for permitting these enolates to react with aldehydes to make the required aldols.
- Aza enolates are the nitrogen analogs of enolates. Imine anions, enamines, metallated Schiff bases, and metalloenzymes are all names for them. Imines are converted into highly nucleophilic aza enolates whenever subjected to treatment with strong bases such as LDA.
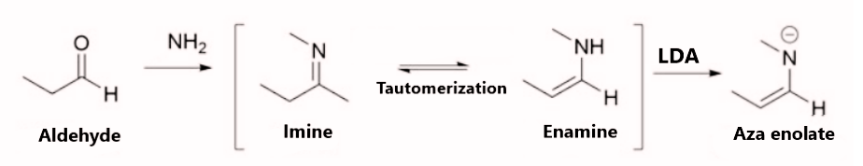
The stability of enolate may be anticipated in the same manner as alkenes can. As a result, the more replaced an enolate is, the more stable it is. However, the more substituted enolate is not always a thermodynamically more stable enolate. This is because, in some situations, steric hindrance destabilizes the more substituted enolate.
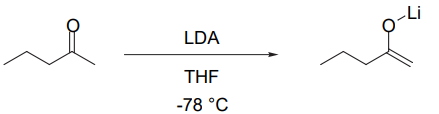
Formation of the Kinetic Enolate
Formation of the Thermodynamic Enolate
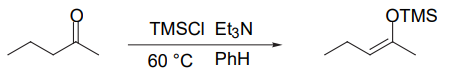
Other Regioselective Enolate Formation Methods
- Enone reduction
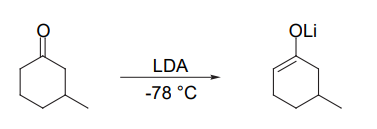
- From α−bromo ketones
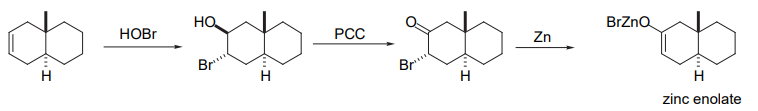
Factors Affecting The Formation of Enolates
The production of enolate is an acid-base process. The following variables can influence the point of equilibrium:
- Solvent
- Base
- Cation
- Temperature
Difference Between Enol and Enolates
Enols
The term ‘enol’ is made up of the words ‘ene’ (as in aldehydes) and ‘ol’ (as in alcohols). They are organic compounds with a hydroxyl (-OH) functional group in direct contact with an alkene (-C=C-) functional group.
Enols are vinyl alcohol compounds with (-C=C-OH) functionality. They are also known as alkenols and are commonly discovered as reaction intermediates in organic chemistry. They are the precursors of enolates and many other chemicals.
Enolates
Enolates are organic anions that are generated from organic molecules, particularly enols. They are most commonly discovered and employed as reagents in the synthesis of organic molecules. Enolates, the most reactive of the three enols, enolates, and enamines, are the most difficult to separate and store.
The interaction of oxygen anion and alkene carbon produces enolates. Enolates are formed during the deprotonation reaction of carbonyl compounds having a hydrogen atom on α-carbon, such as ketones, certain aldehydes, and esters. Because of this, methanal (formaldehyde) cannot form enolates.
Enolates are more reactive than enols because they are conjugate bases of enols, and conjugate bases are always significantly greater nucleophiles than acids themselves.
What is the importance of enolates in organic chemistry?
Enolates are essential in organic chemistry because they may be utilized to generate a wide range of compounds. Enolates can be used to form new bonds, activate or deactivate atom groups, and change the stereochemistry of a molecule. Enolates are also employed in the production of medicines and other chemical substances.
How are enolates stabilized?
Hydrogens, in particular, are mildly acidic because the conjugate base, known as an enolate, is stabilized through conjugation with the orbitals of the carbonyl. The resonance structure of the enolate ion that places the negative charge on the oxygen is the most stable.
Why are enolates more reactive than enols?
Enolates are more flexible and are better nucleophiles than enols due to their negative charges. Because an enolate anion’s negative charge is delocalized between the -carbon and oxygen, electrophiles can connect to either atom.
References
- https://www.pharmacy180.com/article/enolates-1519/
- https://www.quimicaorganica.org/en/organic-synthesis-1/1499-reactions-of-enols-and-enolates.html
- https://www.universalclass.com/articles/science/organic-chemistry/understanding-enols-and-enolates.htm
- https://www.sciencedirect.com/topics/chemistry/enolates#:~:text=Enolates%2C%20formed%20by%20the%20abstraction,order%20primary%20%3E%20secondary%20%3E%20tertiary.
- https://byjus.com/chemistry/enolate/
- https://www.masterorganicchemistry.com/2022/08/16/enolates-properties-reactions/
- https://www.massey.ac.nz/~gjrowlan/stereo2/lecture7.pdf
