An endothermic process absorbs heat and cools the surroundings. The absorbed energy serves as the activation energy for the reaction to take place. As reactants break bonds, energy is released, and when products make new bonds, energy is released. Endothermic processes require the addition of external energy, often in the form of heat, to continue. Since endothermic processes take heat from their surroundings, they tend to chill them. Exothermic reactions are those in which more energy is released when new bonds form in the products than is required to break bonds in the reactants.
What is Endothermic Reaction?
Endothermic reactions are chemical changes that occur when a system absorbs thermal energy from its surroundings, resulting in a rise in its total internal energy level, or Enthalpy. The absorbed energy is retained in the chemical bonds of the products.
The term Endothermic, derived from the French word Endothermique, was coined by Pierre Eugene Marcellin Berthelot while researching the Heat of a Reaction. The name is made up of two basic Greek words: endon (within) and therm (heat).
The following chemical equation represents the endothermic reaction:
Reactants + Energy → Products
Endothermic reactions are chemical processes that make chemicals by absorbing heat from their surroundings. By reducing the temperature of the surrounding environment, these processes produce a cooling effect. Endothermic processes occur when ice cubes absorb heat energy from their surroundings and melt to produce liquid water (no chemical bonds are broken or formed).
When a chemical bond is broken, energy is often released. The formation of chemical bonds requires an energy input as well. Energy may be given or released in a variety of ways (such as heat, light, and electricity). The formation of chemical bonds as a result of heat absorption from the environment characterizes endothermic processes.
Endothermic reactions are characterized as those that absorb heat. When nitrogen and oxygen are heated to roughly 30000 degrees Celsius, they combine to form nitrogen monoxide, absorbing a large amount of heat in the process.

It is an endothermic process because heat is spent in the interaction between nitrogen and oxygen to produce nitrogen monoxide. To indicate an endothermic reaction, put “+Heat” and “+Heat energy” or simply “+Energy” on the reactants’ side of an equation. Nitrogen monoxide is produced by the interaction of nitrogen and oxygen in the air within car engines. All decomposition processes require energy in the form of heat, light, or electricity to occur. Because of this, all breakdown processes are endothermic.
Chemical Processes that are Endothermic
- The interaction of octahydrate crystals of barium hydroxide with dry ammonium chloride
- Ammonium chloride solution in water
- Thionyl chloride (SOCl2) reacts with cobalt (II) sulfate heptahydrate.
- Ammonium nitrate and water are mixed together.
- combining water and potassium chloride
- The reaction between ethanoic acid with sodium carbonate
- Photosynthesis (chlorophyll is utilized to react to carbon dioxide plus water plus energy to generate glucose and oxygen) (chlorophyll is used to react to carbon dioxide plus water plus energy to make glucose and oxygen)
Endothermic Reactions and Energy Change
Endothermic literally means “absorbing heat.” Endothermic reactions require a steady input of energy, generally in the form of heat. This is seen in the diagram below. Because not enough energy is released when the products develop to break additional bonds in the reactants, energy must be continually provided.
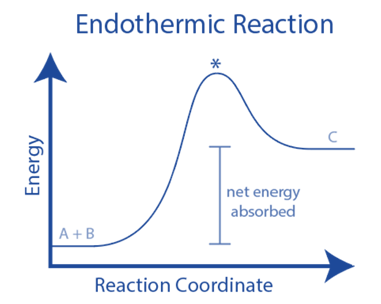
Endothermic processes require the addition of external energy, often in the form of heat, to continue. Since endothermic processes take heat from their surroundings, they tend to chill them.
Endothermic reactions yield products with more energy than the reactants, hence they are frequently non-spontaneous. As a result, the change in enthalpy for an endothermic reaction is always positive.
The change in energy (also known as the enthalpy change and denoted by the symbol ΔH). Endothermic processes add heat to the system as reactants convert to products, hence the sign of enthalpy is positive, i.e. ΔH > 0.
Difference Between an Endothermic and an Endergonic Reaction
Endergonic reactions are nonspontaneous reactions that require energy to occur. Some reactions occur spontaneously, while others require energy from another source to progress. Endergonic reactions are those of the later type that consume energy to go forward. Endergonic means “energy inward,” implying that energy is entering the system.
An endergonic reaction is an endothermic process. Not all endergonic reactions, however, are endothermic. Heat is absorbed in endothermic processes. Sound and light are two more types of energy that might be absorbed in an endergonic process.
Exothermic reactions can be either Endergonic or Exergonic. Scientists utilize the Gibbs free energy change equation to forecast this. This equation links the free energy (G), enthalpy (H), and entropy (S) of a system at a given temperature as follows:

Gibbs free energy is the energy that can be transformed into productive work inside a system. When working on the System ΔG, it has a positive value. Endothermic systems always have a positive ΔG value because they produce work when energy is introduced to them. An Endothermic reaction is Exergonic, or spontaneous, when the Entropy change is positive (ΔS > 0), and Endergonic, or nonspontaneous, when the Entropy change is negative (ΔS< 0)
To put it simply, Endothermic refers to thermal energy movement, whereas Endergonic refers to spontaneity. The free energy of the system (ΔG > 0) increases in both spontaneous and non-spontaneous endothermic processes.
Read Also: Difference Between Endothermic and Exothermic Reactions
Endothermic Reaction Examples
The human body employs the endothermic property of evaporation to stay cool. Sweating is one method for attaining this. Sweat (which forms on the surface of the skin) absorbs heat from the skin and evaporates, resulting in a cooling effect.
Sweating, on the other hand, is an endogenous reaction. In a chemical reaction, existing chemical bonds can be broken, new chemical bonds can be created, or both can occur. Sweat evaporation causes no chemical changes, but it does cause a physical phase shift (from liquid to vapour). As a result, evaporation is categorized as a physical response rather than an endothermic.
These examples might be described as chemical reactions, but they are more commonly thought of as endothermic (heat-absorbing) processes:
- Melting ice cubes
- Solid salts melting
- The process of evaporating liquid water
- Transforming frost to water vapor (in general, melting, boiling, and evaporation are endothermic reactions).
- Anhydrous salt formation from a hydrate
- converting an atom in the gas phase into a cation
- The splitting of a gas molecule
- Ion pair separation
- Making an egg
- Making bread
Endothermic Reaction with Chemical Equations
Calcium carbonate, for example, decomposes into calcium oxide and carbon dioxide when heated.

- The breakdown of calcium carbonate is an endothermic process because heat energy is absorbed.
- Photosynthesis is an endothermic process. This is because green plants absorb solar energy during the photosynthesis process.
- An endothermic process occurs when water electrolysis creates hydrogen and oxygen. This is because electric energy is absorbed during this process. This talk makes it clear that energy may be given out or absorbed in chemical processes in the form of heat, light, or electricity.
Photosynthesis is one of the most significant endothermic processes. Plants produce the basic sugar glucose (C6H12O6) from carbon dioxide (CO2) and water during photosynthesis (H2O). In the process, they also emit oxygen (O2). This chemical equation summarizes photosynthetic reactions:

Light provides the energy for photosynthesis. Photosynthesis cannot proceed in the absence of light energy.
NaCl dissolution: Sodium chloride, or ordinary table salt, dissolves endothermically. In other words, if you dissolve it in water, the water will become somewhat cooler due to the energy absorbed by the ion solvation.
NaCl → Na+ (aq) + Cl– (aq)
This, however, is not always true for all salts. Dissolution can be both endothermic and exothermic depending on the chemical dissolved.
References
- Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015). Principle of Modern Chemistry, Brooks Cole. p. 617. ISBN 978-1305079113
- “Exothermic & Endothermic Reactions | Energy Foundations for High School Chemistry”. highschoolenergy.acs.org. Retrieved 2021-04-11.
- Helmenstine, Anne Marie, Ph.D. “Examples of Endothermic Reactions.” ThoughtCo, Sep. 7, 2021, thoughtco.com/endothermic-reaction-examples-608179.
- Austin, Patrick (January 1996). “Tritium: The environmental, health, budgetary, and strategic effects of the Department of Energy’s decision to produce tritium”. Institute for Energy and Environmental Research. Retrieved 2010-09-15.
- https://www.thoughtco.com/endothermic-reaction-examples-608179
- https://en.wikipedia.org/wiki/Endothermic_process
- https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/17.4/primary/lesson/endothermic-reactions-ms-ps/
- https://byjus.com/chemistry/endothermic-reaction/
- https://chemistrytalk.org/endothermic-vs-exothermic-reactions/
- https://study.com/learn/lesson/endothermic-reaction.html
- https://www.geeksforgeeks.org/difference-between-exothermic-and-endothermic-reactions/
