The main difference between enantiomers vs diastereomers is that enantiomers are found as mirror images whereas diastereomers are not.
Enantiomers and diastereomers are two different types of stereoisomers. Stereoisomers are groups of molecules with identical connectivity and chemical composition but differing spatial arrangements of their atoms. Enantiomers and diastereomers are also referred to as optical isomers because of how they interact with light. Each molecule in an enantiomer or diastereomer pair causes polarized light to bend in the opposite direction.
Enantiomers and Diastereomers are the two types of stereoisomers. Enantiomers arise as a result of chirality. It is a molecule with a single atom and four substituents that produce enantiomers. They are mirror reflections of one another. A racemic mixture is formed when the two enantiomers have the same quantities. Diastereomers, on the other hand, have ring structures that are chemical compounds with the same molecular formula. They are not mirror reflections of one other. They can have many chiral centers. They have various melting points, boiling points, and densities.
Interesting Science Videos
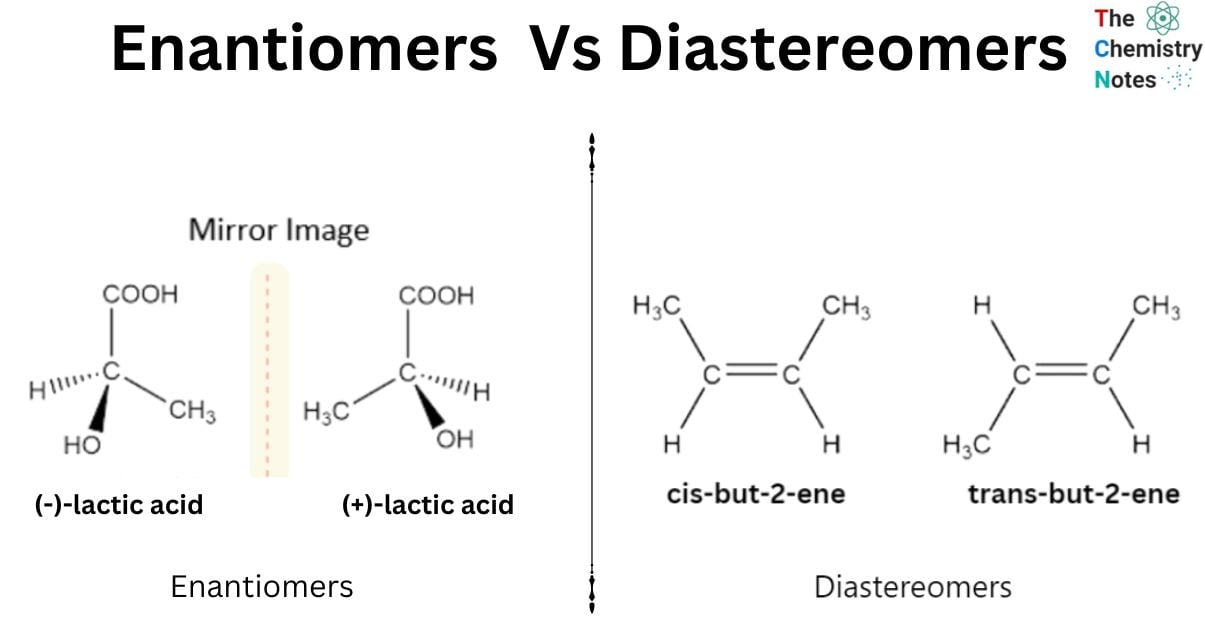
Enantiomers
- Enantiomers are stereoisomers, which means that they have the same connectivity but a different spatial orientation than other molecules.
- There are differences between the chiral and enantiomer groups. Enantiomers, unlike chirals, have two distinct forms that are not superposed on one another.
- Their arrangements change at locations known as chiral centers, which are made up of one central atom coupled to four different atoms, or groups of atoms, that are all different.
- Enantiomers of a molecule require at least one chiral center. Enantiomers include molecules A and B, which are the same as each other. Both times, the central carbon functions as a chiral center.
- Enantiomers occur in pairs, with the two molecules classified as R-enantiomers or S-enantiomers. They have the same physical and chemical characteristics. Lactic acid, amino acids, D-alanine, and other substances are examples.
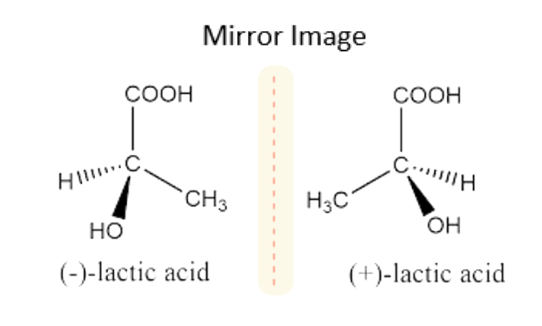
Diastereomers
- Diastereomers contain ring structures, which are chemical compounds with the same molecular formula, and they are non-mirror images. These items cannot be superimposable.
- They include cis-trans isomers, stereoisomers, and double-bond isomers. They can have more than one chiral center.
- They have variable melting and boiling temperatures, as well as densities. They are optically inactive. They do not have an identical rotational angle.
- Diastereomers can be separated via chromatography, fractional crystallization, and fractional crystallization.
- They have several R and S arrangements in one stereocenter. For example, tartaric acid, chlorine atoms, ethyl, and so on.
Differences Between Enantiomers and Diastereomers
| Enantiomers | Diastereomers |
|---|---|
| Enantiomers are pair of molecules that exist in two forms that are mirror copies of one another but cannot be overlaid one on the other. | Diastereomers are substances that have the same molecular formula and arrangement of bonded elements as other substances but are not mirror or superimposable copies of one another. |
| Enantiomers share the same physical and chemical properties. | Diastereomers has the distinct physical and chemical properties. |
| Enantiomer has identical molecular shapes. | The molecular shapes of a diastereomer are distinct. |
| The molecules exist in paired form. | The molecules exist in isolation. |
| Polarized light can be used to differentiate them. | They may be recognized by their lack of mirror images and difference in stereoisomers. |
| Enantiomers have equal but diametrically opposed rotational axes. | Contrary to enantiomers, diastereomers have an identical angle of rotation. |
Similarities Between Enantiomers and Diastereomers
- Enantiomers and diastereomers are both stereoisomers.
- Both have chiral centers and are optically active.
- Enantiomers and diastereomers both are non superimposable.
Video Reference
References
- https://www.masterorganicchemistry.com/2019/03/08/enantiomers-diastereomers-or-the-same-1-using-models/
- https://chemistrytalk.org/enantiomers-diastereomers/
- https://www.geeksforgeeks.org/difference-between-enantiomers-and-diastereomers/
- https://openpress.usask.ca/intro-organic-chemistry/chapter/4-6/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Chirality/Chirality_and_Stereoisomers#:~:text=Stereoisomers%20are%20isomers%20that%20differ,mirror%20image%20of%20one%20another.

