Electrophoresis is a method of separating molecules on the basis of their ability to move in an electric field. Electrophoresis has become the most extensively used method for analyzing biomolecules in biochemistry or molecular biology, including genetic components such as DNA or RNA, proteins, and polysaccharides.
Interesting Science Videos
Principle of electrophoresis
When a potential difference is introduced, molecules with different overall charges start to segregate due to differences in electrophoretic mobility. Even molecules with equal charges will begin to split if their molecular sizes differ because they would encounter distinct frictional forces. As a result, certain forms of electrophoresis rely almost entirely on the various charges on molecules for separation, whilst others rely on differences in the size (molecular size) of molecules.
The migration and separation of charged particles (ions) under the influence of an electric field are referred to as electrophoresis. An electrophoretic system consists of two electrodes of opposite charge (anode, cathode) linked by a conducting substance known as an electrolyte. The separation effect on ionic particles is caused by changes in velocity (v), which is the product of particle mobility (m) and field strength (E):
V = ME
An ionic particle’s mobility (m), which is constant under specific electrophoretic circumstances, is dictated by the particle’s size, shape, charge, and temperature during separation.
The rate of movement of charged molecules is affected by the following factors:
(a) The electric field’s strength, size, and shape.
(b) The sample’s relative hydrophobicity.
(c) The buffer’s ionic strength and temperature.
(d) The biomolecule’s molecular size.
(e) The biomolecule’s net charge density.
(f) The biomolecule’s shape.
Types of electrophoresis
Electrophoresis is the movement of charged molecules through liquids in an electric field. Electrophoresis is frequently characterized based on the presence or absence of a solid support medium or matrix through which the charged molecules in the electrophoretic solution migrate.
Electrophoresis can be categorized into two types based on the presence or absence of supporting media:
- free electrophoresis
- zone electrophoresis.
Free electrophoresis
Aqueous buffers are used in solution electrophoresis systems. Such systems may experience sample mixing mainly as a result of charged molecule diffusion, resulting in a loss of resolution during the sample application, separation, and removal steps. As a result, solution electrophoresis systems must employ some method of aqueous solution stabilization in the electrophoresis cell. To minimize diffusional mixing of the materials being separated during electrophoresis, soluble-gradient electrophoresis systems, for example, use variable densities of a non-ionic solute (e.g., sucrose or glycerol). Despite these improvements, solution electrophoresis devices only have limited uses, typically where preparative scale electrophoretic separation is required.
Zone electrophoresis
Zone electrophoresis is similar to moving boundary electrophoresis in that it uses a homogeneous buffer solution to separate proteins. This format usually employs a support medium or matrix to decrease convection current and avoid uncontrolled sample diffusion. In most cases, the matrix has an additional sieving action that affects electrophoretic separation. Samples for ZE separation are enclosed in an electrophoretic solution buffer and separated in the matrix for a predetermined amount of time. When an electric current (q) is applied to the sample, it moves at different speeds depending on its mass and charge. After the separation operation is completed, components of the sample with comparable qualities are divided into a separate zone.
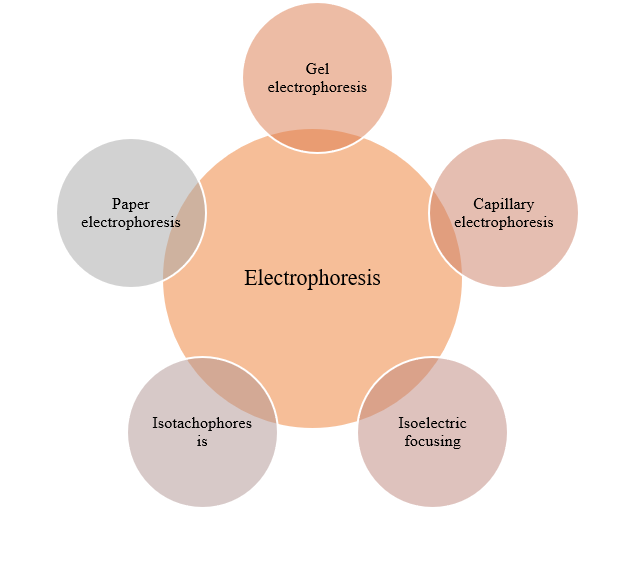
There are mainly four types of zone electrophoresis:
(a) paper electrophoresis,
(b) cellulose acetate electrophoresis,
(c) capillary electrophoresis,
(d) gel electrophoresis.
Paper electrophoresis
In this type of electrophoresis, a filter paper (similar to chromatography paper) with a low adsorption capacity and homogeneous pore size serves as the supporting medium for sample separation under the effect of an applied electric field. A strip of filter paper is wet with buffer before being immersed in buffer reservoirs containing the electrodes during paper electrophoresis.
The samples are placed in the center of the paper, a high voltage is delivered, and the spots move based on their charges. Depending on their chemical identity, the separated components can be recognized using a variety of staining procedures after electrophoresis.
Cellulose acetate electrophoresis
It is a modified form of paper electrophoresis, which Kohn invented in 1958. Bacteriological acetate membrane filters are used instead of ordinary chromatography paper in this sort of electrophoresis. The advantages of cellulose acetate strips over chromatography paper are as follows:
(a) The cellulose acetate strips are chemically pure and devoid of lignin and hemicelluloses, and they serve as barriers in the free moment of big molecules in general.
(b) Due to the low content of glucose cellulose acetate strips, it is ideal for polysaccharide electrophoresis.
(c) Since cellulose acetate is not hydrophilic, it holds very little buffer, allowing for faster resolution.
Gel electrophoresis
Gel electrophoresis is a technique for separating DNA fragments (or other macromolecules like RNA and proteins) depending on size and charge. Electrophoresis is the process of passing a current across a gel containing the molecules of interest. The molecules will go through the gel in different directions or at different speeds depending on their size and charge, allowing them to be segregated from one another.
Agarose gel electrophoresis
Agarose gel is used as the supporting medium in this form of electrophoresis. This is used for nucleic acid electrophoresis, such as DNA and RNA.
When a potential difference is applied across the electrodes of an agarose gel-filled horizontal electrophoretic tank and biomolecules are loaded, they are separated based on their molecular size and move to their respective electrodes. The agarose gel works as a sieve in this case.
In the same way that large particles remain above a sieve while particles smaller than the pore size passes through it, larger and bulky molecules remain behind while smaller molecules flow faster and more swiftly toward their respective electrodes.
Smaller proteins migrate quicker due to less resistance from the gel matrix when separated by electrophoresis across a gel matrix. The structure and charge of the proteins also affect the rate of migration across the gel matrix.
SDS-PAGE electrophoresis
The use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel in SDS-PAGE greatly reduces the influence of structure and charge, and proteins are separated based on polypeptide chain length.
SDS is a detergent that binds to the protein backbone at a constant molar ratio and has a significant protein-denaturing effect. Proteins unfold into linear chains with negative charge proportionate to polypeptide chain length in the presence of SDS and a reducing agent that cleaves disulfide bonds required for optimal folding.
Capillary electrophoresis
The narrow bore tube’s capillarity is used to separate the samples depending on their size: charge ratio. In comparison to established separation techniques such as agarose gel electrophoresis or SDS-PAGE, capillary electrophoresis (CE) is a relatively new separation technique. It has exceptional qualities that make it both competitive and a good option. The capacity to separate charged and non-charged molecules is one of the key advantages of capillary electrophoresis over other separation techniques. CE separates analyte ions in an electrolyte solution (background electrolyte) within a tiny fused silica capillary.
Affinity electrophoresis
Affinity Electrophoresis is a type of electrophoresis that separates a biomolecule that interacts with and binds to another molecule for which it has an affinity. It makes use of the fact that when a biomolecule binds to another molecule, its electrical mobility changes and this change in electrical mobility is recorded in the electrophoretic pattern.
Other electrophoresis technique
Isotacho electrophoresis and isotachophoresis
In isotachophoresis, often known as ITP, all ions flow at the same pace. In this process, a leading or terminating electrolyte is sandwiched between two non-homogeneous solution buffers. Both electrolytes charge particles in an interest sample in the same way. An electric current will have a greater impact on a leading electrolyte than a charged particle in the sample or even a terminating electrolyte. Charged particles will be displaced during the ITP, lowering electrical mobility and resulting in a continuous area of charged particles with equivalent properties wedged between regions occupied by the leading and terminating ions during the process.
Isoelectric focusing
Isoelectric focusing is a technique for separating proteins depending on their net charge, commonly known as the protein’s isoelectric point. This is accomplished by immersing the protein sample in a pH gradient slab created by an electric field. This causes the proteins to migrate down the pH gradient field until they reach a pH where their isoelectric point is zero.
Immunoelectrophoresis
Immunoelectrophoresis is a type of electrophoresis in which antigens, such as proteins or peptides, are separated based on their interaction with antibodies or immunoglobulins, or their specificity (Ig). When an antigen and its corresponding antibody bind at a given antigen/antibody ratio, or even at the equivalent point, the antigen-antibody complex precipitates.
Factors affecting electrophoresis
Sample
The migration rate of the sample being separated is affected by its charge, size, and shape. A net increase in the charge accelerates migration. The rate of migration is determined by molecule size (inversely proportional) and sample shape.
Buffer solution
Buffer influences compound migration rate and stabilizes the pH of the supporting medium. It has been discovered that zwitterionic buffers may resist continuous electrolysis far better than standard buffers, particularly in capillary zone electrophoresis.
Frictional force
A frictional force also slows the mobility of this charged molecule. This frictional force is a measure of the molecule’s hydrodynamic size, shape, the pore size of the medium in which the electrophoresis is taking place, and the viscosity of the buffer.
Applied voltage
The voltage applied influences the travel time of the molecules being separated. The higher the voltage, the faster the DNA will flow through the gel. However, excessive voltages may melt the gel or produce smearing or distortion of DNA bands.
Supporting media
The rate of compound migration is affected by the type of supporting media used. It is always preferable to use inert media. Adsorption, molecular sieving, and electro-osmosis processes may occur in the medium, affecting the electrophoretic rate. The sample moves like a comet rather than a band as a result of tailing caused by adsorption. This decreases both the rate and the resolution of the separation.
Electroendosmosis
Electroendosmosis (also known as electro-osmotic flow) is an important factor that might alter electrophoretic separation.
The presence of charged groups on the surface of the support medium causes this behavior. Paper, for example, includes some carboxyl groups, agarose contains sulfate groups depending on the purity grade, and the surface of glass walls used in capillary electrophoresis contains silanol (Si-OH) groups.
At the suitable pH, these groups will ionize, resulting in charged sites. These charges are responsible for electroendosmosis. In the case of capillary electrophoresis, the ionized silanol groups form an electrical double layer, or a charge separation area, at the capillary wall/electrolytic contact.
When a voltage is given to the electrolyte near the capillary walls, cations in the electrolyte migrate toward the cathode, dragging the electrolyte solution with them. This results in a net electroosmotic flow towards the cathode.
Applications of electrophoresis
DNA analysis
One of the most common uses for electrophoresis is DNA analysis. Researchers can divide DNA into segments using an electrical charge and maintain the molecules in place once the charge is withdrawn using the gel as a medium. This enables researchers to examine molecules at high resolutions, making full analysis of DNA architecture much easier.
Explosives chemical and residue analysis
Capillary electrophoresis is employed in the trace analysis of organic and inorganic gunshot residues and explosives. Organic gunpowder additives such as ethylcentralite, diphenylamine, and nitroglycerin can be examined using Miceller Electro kinetic capillary chromatography.
Protein detection
Immunoelectrophoresis is a sort of electrophoresis that is commonly used to examine the existence of various types of proteins and how they react chemically in different settings. When abnormal protein molecules form, they become stimulated by several medical problems such as multiple sclerosis, kidney failure, and even various types of cancer. The irregular proteins are detected using electrophoresis on urine or blood samples and the results are constantly monitored for any variations from typical protein shapes and levels. Immunoelectrophoresis is also used to identify particular proteins known as immunoglobulins.
Analysis of drug abuse:
One-of-a-kind organic samples, for example, tissue, hair, nail, and body fluids, are often used in the detection of illicit tablets. Capillary electrophoresis is used to detect drugs.
Test for antibodies
When it comes to antibiotic testing, electrophoresis serves several important roles.
The testing of antibiotics to ensure their purity is one of the most prevalent applications of the electrophoresis technique in this industry. Electrophoresis is utilized in a solution containing the antibiotic to be tested on a paper strip. This strip is impregnated with a capillary or antibiotic that contains the medicine.
Electrophoresis is also used to determine the potency of the antibiotic, which is critical in providing the correct dosages. In addition, the antibiotic research and genetic testing fields share a common ground. As a result, electrophoresis aids in the identification of genes that signal resistance to a specific type of antibiotic.
Vaccine testing
Electrophoresis has been critical in the creation of modern vaccines. it is used to test vaccine quality and concentration. To determine the best potential form of a single vaccine, researchers utilize electrophoresis to evaluate different varieties of vaccines with varying quantities and types of antibodies.
Advantages of electrophoresis
- It has a high efficiency of separation.
- It provides sample analysis in a short period of time.
- It produces fewer waste products.
- It is a simple strategy to use.
- The experiment can be performed with a small amount of sample.
Disadvantages of electrophoresis
- During electrophoresis, gels can melt, the buffer can run out, and various genetic materials can run in unanticipated manners.
- Heat is dissipated by the capillary tube’s narrow diameter, resulting in greater diffusion. As a result, the resolution is not always accurate.
References
- https://www.jetir.org/papers/JETIRFH06051.pdf
- https://www.vedantu.com/chemistry/application-of-electrophoresis.
- https://uomustansiriyah.edu.iq/media/lectures/4/4_2021_09_14!07_58_34_PM.pdf.
- https://soe.unipune.ac.in/studymaterial/ashwiniWadegaonkarSelf/BSC%20821%20Ch%205.pdf.
- Ferrier D. R. (2017). Lippincott illustrated reviews : biochemistry (Seventh). Wolters Kluwer.
- https://ulbld.lf1.cuni.cz/file/4576/electrophoresis-in- biochemistry.pdf
