Interesting Science Videos
Introduction to Electrolysis
Electrolysis is the process of breaking down a chemical complex into its component parts or other compounds by exposing it to an electric current
Electrolysis is made up of the phrases “electro” (electricity or electrons) and “lysis” (breaking apart). Ions migrate during this procedure. Since they are bound together by strong ionic forces, salts do not contain mobile ions in their solid state. High temperatures, however, cause them to disintegrate (in their molten form) into their corresponding mobile ions. Therefore, electrolysis of molten salts refers to a procedure where electricity is transferred through a salt’s liquid (molten) form while it contains ions to break it down into its component elements.
Many bulk chemicals with industrial use, such as sodium hydroxide and chlorine, are created by an important process known as sodium chloride electrolysis. Sodium chloride is electrolyzed either as a liquid or after it has been dissolved in water. In electrolysis, additional salts are introduced to help the redox reactions.
When we electrolyze, we often do a nonspontaneous endothermic reaction in which the products are less stable than the reactants. The battery or power source that powers the redox process provides the energy difference.
Electrolyte
- Electrolytes are substances which release ions when dissolved into water.
- Because all of them produce ions when dissolved in water, they may be classified as acids, bases, and salts.
- Due to the mobility of the positive and negative ions, known as cations and anions, respectively, these solutions transmit electricity.
- Strong electrolytes do not generate any neutral molecules in solution; instead, they entirely ionize when dissolved.
- For instance, strong electrolytes include NaCl, HNO3, HClO3, and CaCl2.
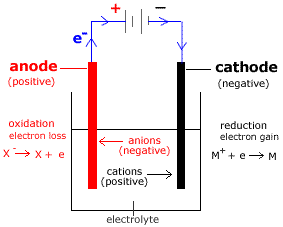
Electrode
- A solid electric conductor known as an electrode is used to introduce electric current into non-metallic solids, liquids, gases, plasmas, or vacuums.
- Electrodes do not require the fabrication of metal in order to be efficient electrical conductors.
- There are two types of electrode
- Cathode: The cathode is the electrode that undergoes reduction.
- Anode: The anode is the electrode where oxidation occurs.
Electrolytic Cell
- Molten salts are electrolyzed in an electrolytic cell. In an electrolytic cell, electrical energy is changed into chemical energy.
- A non-spontaneous reaction (chemical energy) is possible thanks to the battery’s electric energy.
- The electrodes in electrolytic cells are always either positive or negative since they are powered by direct current.
- It would be challenging to collect the byproducts of decomposition from just the anode or cathode if you utilized an alternating current power source since the electrodes would constantly switch from positive to negative.
Electrolytic Solution
- It is the combination of electrolytically active mobile ions. Alternately, the salt’s liquid state (in the case of electrolysis of liquid salts) or its aqueous solution (in the case of electrolysis of an aqueous salt solution).
- To create the products, we need a constant flow of ions at each electrode inside the electrolytic cell. If the ions can’t migrate, then this won’t happen.
- The most straightforward method for making ions mobile is to turn them into a liquid.
A salt can be much harder to melt than it appears in theory. Because of the strong ionic interactions between their cations and anions, salts are ionic compounds with exceptionally high melting temperatures.
Here are some common salts’ melting points.
| Salt | Anion | Cation | Melting Point (°C) |
|---|---|---|---|
| Magnesium Chloride (MgCl2) | Mg2+ | Cl– | 714 |
| Sodium Chloride (NaCl) | Na+ | Cl– | 801 |
| Lithium Chloride (LiCl) | Li+ | Cl– | 605 |
| Calcium Chloride (CaCl2) | Ca2+ | Cl– | 772 |
Electrolysis of Sodium Chloride: Molten and Aqueous Salts
Electrolysis of Molten Salts (Sodium Chloride)
Ions are moved to an electrode during electrolysis. Ions cannot migrate in solid state, making it unsuitable for electrolysis. When melted at high temperatures, sodium chloride splits into sodium and chloride ions, allowing electrolysis to produce sodium atoms and chlorine gas.
Na + (l) + Cl- (l) = NaClAt the cathode, 2Na+(l) + e– are reduced to Na (l).
2Na+(l) + e– → Na (l)At the anode, 2Cl– (l) is oxidized to Cl2 (g) and 2e–
2Cl– (l) → Cl2 (g) + 2e–The formula for the net reaction is:
2Na+ (l) + 2Cl– (l) → 2Na (l) + Cl2 (g)Down’s Process
- The melting point of sodium chloride is 801 °C, which is quite high.
- The addition of anhydride calcium chloride in the proportion CaCl2: NaCl = 3:2 lowers the melting point to 580°C.
- Iron cathode and graphite anode are used in electrolysis, and iron gauze is used to stop the products, chlorine and sodium, from combining.
- Chlorine gas and sodium metal are the byproducts of molten sodium chloride.
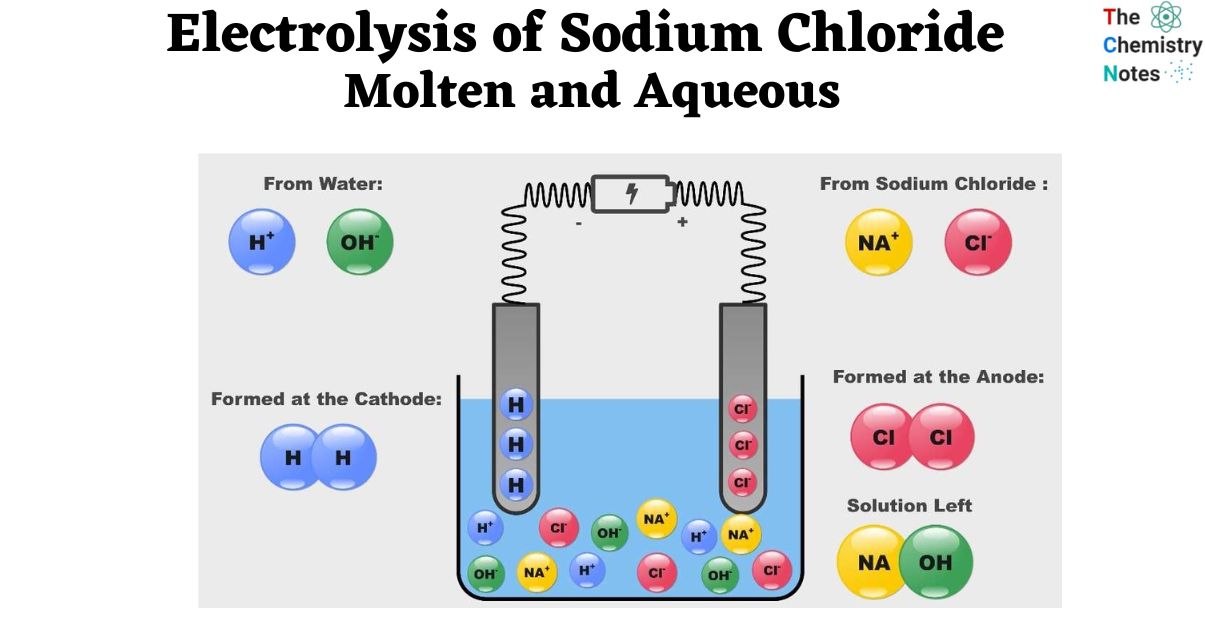
Electrolysis of Aqueous Sodium Chloride
- In an aqueous solution, sodium chloride is split into sodium and chloride ions. In an aqueous solution, sodium chloride may be electrolyzed more easily.
- However, at various potentials, water may also experience reduction and oxidation events.
- Therefore, the material that is oxidized or reduced may not only consist of sodium and chloride ions but also include the water molecule.
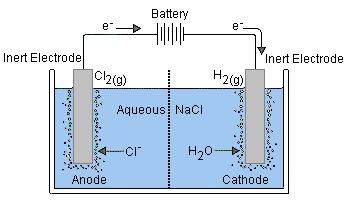
Two competing reactions are feasible at both the cathode and anode,
At Cathode
- At cathode the reduction process occurs at a pH of 7.
- Water can be converted to hydrogen gas, and sodium ions can be converted to sodium metal.
2H2O (l) + 2e– → H2 (g) + 2OH– E° = -1.0 VNa+ (l) + e– → Na (l) E° = -2.71VAt Anode
- At the anode, the oxidation process occurs at a pH of 7.
- Water may be oxidized to produce oxygen, or chloride ions can be oxidized to produce chlorine molecules.
2H2O → O2 (g) + 4H+ + 4e– E° = -1.42 V2Cl– → Cl2 + 2e– E° = - 1.36VConsequently, the end result of electrolysis of aqueous sodium chloride might range from,
- At the cathode, sodium metal or hydrogen gas
- Gas, either chlorine or oxygen, near the anode
- Sodium hydroxide is a byproduct of the reaction between sodium and water, and the outcome of electrolysis depends on the amount of Sodium Chloride (NaCl) present in the aqueous solution.
Product of Electrolysis of Molten Salts
Sodium Bromide (NaBr)
- At anode: Bromine gas
- At cathode: Sodium deposition
Copper(II) Chloride (CuCl2)
- At anode: Chlorine gas
- At cathode: Copper deposition
Zinc Chloride (ZnCl)
- At anode: Chlorine gas
- At cathode: Zinc deposition
The material deposited at electrodes and the amount of electric current flowing are connected, as demonstrated by Faraday’s Laws of Electrolysis.
- First Law: According to the first rule, the quantity of electric current flowing is exactly proportional to the weight of the material deposited at an electrode.
- Second Law: The second law asserts that the weight of the material deposited at an electrode is directly proportionate to the chemical equivalent of the substance when the same amount of electric current is transmitted through various electrolytic solutions.
What is the Difference Between Molten and Aqueous Electrolysis?
Molten and aqueous electrolysis are chemistry analytical procedures for separating chemical elements in an analyte sample. The primary distinction between molten and aqueous electrolysis is that molten electrolysis yields analyte elements, whereas aqueous electrolysis yields an aqueous salt solution and a gas mixture as the end result.
Major application of electrolysis of molten electrolysis is in extraction of aluminum and application of electrolysis of aqueous electrolysis is in electrolysis of table salt.
Conclusion
An electric current is sent through molten salts during the electrolysis process to break them down into their chemical components. There is no need for a salt bridge because it occurs in an electrolytic cell. Positive charge surrounds the anode. There is a negative charge on the cathode. When salts are electrolyzed when in a molten state, we get different results than when we electrolyze the salts in an aqueous solution. It serves as the foundation for many industrial procedures that purify impure, highly reactive materials. Examples include sodium, copper, and so on.
Frequently Asked Questions (FAQ)
What is an Electrolysis?
Electrolysis is a process in which an electric current is conducted through a liquid or solution containing ions, causing the compounds inside to disintegrate.
What is the difference between electrolysis of molten sodium chloride and aqueous sodium chloride?
Aqueous sodium chloride solution electrolyzed produces chlorine gas at the anode and hydrogen gas at the cathode. Whereas electrolysis of molten sodium chloride yields chlorine gas at anode but liquid sodium metal at cathode.
Video References
References
- https://www.chemguide.co.uk/inorganic/electrolysis/melts.html
- https://www.bbc.co.uk/bitesize/guides/zhk6pbk/revision/2#:~:text=New%20substances%20form%20when%20a,Bunsen%20burner%20until%20it%20melts
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK12)/23%3A_Electrochemistry/23.10%3A_Electrolysis_of_Molten_Salts_and_Electrolysis_of_Brine
- https://www.ausetute.com.au/elymsalt.html

