In the modern world, it is challenging to get through a single day without interacting with electrochemistry. An electrochemical reaction provides the necessary energy for any device that requires a battery. Around us, there is a sizable “electric world.” Electricity is essential to our society. Additionally, we live in a vast “chemical world.” These two worlds are united by electrochemistry, which connects them.
Both theoretical and practical considerations make the topic important. Electrochemical processes are used to produce a large number of metals, sodium hydroxide, chlorine, fluorine, and many other chemicals. Chemical energy can be converted into electrical energy by batteries and fuel cells, which are widely used in a variety of tools and appliances.Electrochemical reactions have the potential to be less destructive and energy-intensive.
Therefore, electrochemical research is crucial for developing new environmentally friendly technologies.
Electrochemistry- Introduction
Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well as the application of electrical energy to non-spontaneous chemical transformations.
Chemistry’s subfield of electrochemistry focuses on understanding how electrical energy and chemical changes interact. The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element to another in a process known as an oxidation-reduction (“redox”) reaction, is the result of this electron movement. Electrochemical reactions are those in which electric currents are either generated or input. These responses can be broadly divided into two categories:
- electrical energy produces a chemical change
- chemical energy to electrical energy conversion
When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemistry typically deals with the overall reactions that occur when several redox reactions occur at the same time. Also they are linked by an appropriate electrolyte and an external electric current. In other words, electrochemistry also addresses chemical phenomena related to charge separation (as seen commonly in liquids such as solutions). Charge transfer between various chemical species, whether homogeneously or heterogeneously, frequently occurs during the dissociation of charge.
Electrolytic Cells
Electrolysis is the breakdown of a compound into its constituent parts using an electric current. A compound that is either molten or in the solution can undergo electrolysis, which is a chemical transformation.
It is frequently employed to extract metals with a high reactivity series. It is impossible to extract the metals from the ores of these metals by heating them with carbon. In addition to producing non-metals like chlorine, electrolysis also helps to clean up some metals. Typically, electrolysis takes place in an electrolysis cell.
In many metallurgical processes, electrolysis is applicable, such as in the electrowinning or electrorefining of metals from ores or compounds and in the deposition of metals from solution (electroplating). It is possible to electrolyze molten sodium chloride to produce metallic sodium and chlorine gas as well as sodium hydroxide and chlorine gas from an aqueous solution of sodium chloride. Hydrogen and oxygen are produced electrochemically from water.
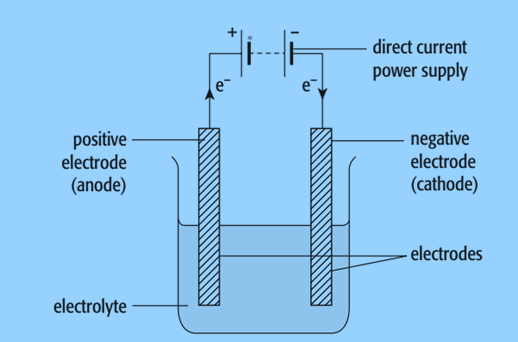
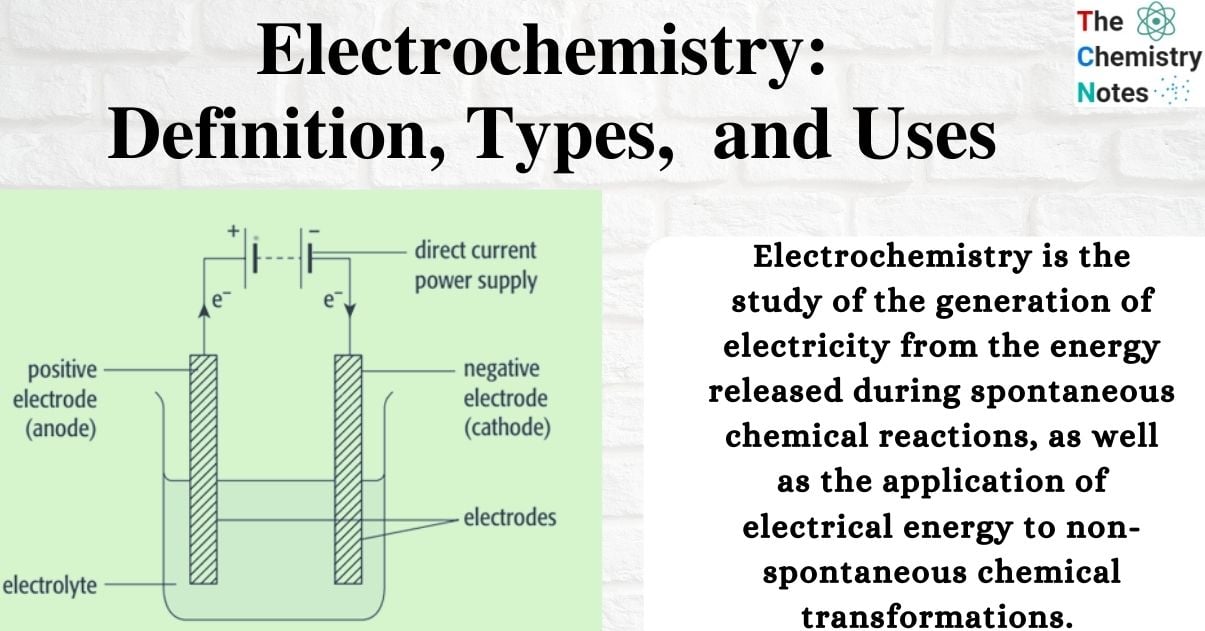
Components of Electrolysis Cell
Electrolyte: The electrolyte, which is the substance that breaks down in the electrolysis cell, can either be a molten ionic compound or a concentrated aqueous solution of ions.
Electrodes: The electrodes, which are rods made of metal or carbon (graphite), conduct electricity to and from the electrolyte.
- Anode: The positive electrode
- Cathode: The negative electrode
Direct current: The power supply must be direct current.
Redox reactions in electrolysis
A compound’s element’s oxidation number essentially measures how its atoms’ electronic environment differs from that of the element’s pure atoms. The charge on a molecule in ionic compounds is equal to the sum of the oxidation numbers of all the atoms in the molecule. When exposed to suitable solvents (typically water), ionic compounds usually separate into positive and negative ions, making them available for redox reactions and electrochemical applications since the solvent serves as the medium for the flow of the electric current. Water or its characteristic ions, H+ (aq) and OH– (aq), act as reactants or products in redox reactions in aqueous solutions.
The positive ions (cations) move to the cathode during electrolysis. They receive electrons from the cathode when they arrive.
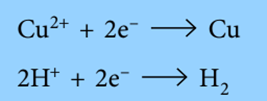
If metal atoms are created, they might be deposited on the cathode as a layer of metal. Alternatively, they might melt into a layer inside the cell. Hydrogen gas bubbles off when it is created.
The anode receives the negative ions (anion). They lose electrons to the anode when they get to the anode.
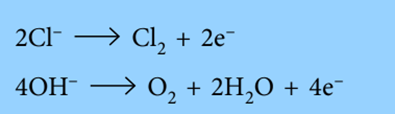
Oxidation is the loss of electrons. Oxidation always takes place at the anode.
Hence, electrolysis is a redox reaction
For example: the electrode reactions when electrolyzing molten zinc chloride is:
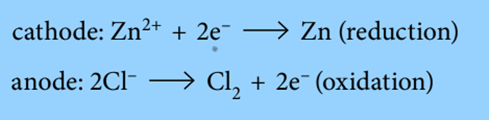
The electron gain at the cathode counteracts the electron loss at the anode. Overall, the reaction is:
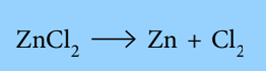
Electrochemical Cell
The Gibbs free energy of a system decreases during a spontaneous chemical reaction. In electrochemistry, redox reactions that occur spontaneously transform chemical energy into electrical energy. A non-spontaneous chemical reaction can also happen oppositely by applying electricity. Electrochemical cells are the pieces of machinery applicable in carrying out these interconversions.
All the reactants and products of a redox system are in well-known electrochemical cells, which forbid physical contact between the reactants. As a result, electrons cannot transfer directly; instead, they can only transfer indirectly by coming into contact with the separated reactants through an external circuit. Voltaic or galvanic cells are common names for these components.
An electrochemical cell is a device or apparatus that generates electric current from chemical change and the energy released by this spontaneous redox reaction. An electric current is created when electrons are transferred from one chemical species to another. Chemical energy transforms into electrical energy and vice versa by electrochemical cells.
Two half-cells make up an electrochemical cell. Each of them consists of an electrode and an electrolyte, which may or may not be the same in the two half-cells.
Components of Electrochemical Cells
The main components of an electrochemical cell are:
Electrodes – The solid electrical conductors found in electrochemical cells made of good conductors like metals. They are of two categories:
a. The Anode – The cell compartment in which oxidation occurs.
b. The Cathode- The cell compartment where reduction occurs.
Electrolyte: The term “electrolyte” refers to a substance that lies between the electrodes and, when dissolved in polar solvents like water, produces freely moving ions that form an electrically conducting solution.
External Circuit: The external circuit enables the movement of electrons from the anode to the cathode. This spontaneous flow can generate energy that can produce heat or perform work in the case of a galvanic cell. This needs an energy source to function in the case of an electrolytic cell.
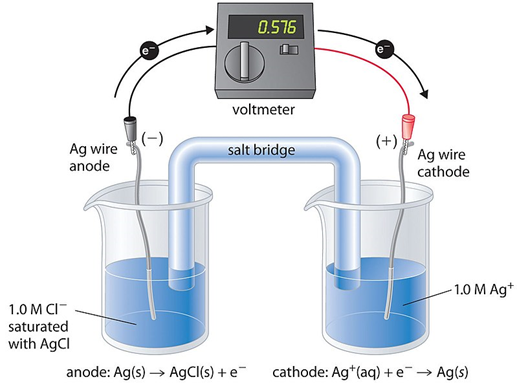
Salt Bridge: An electrochemical cell’s oxidation and reduction halves are connected by a salt bridge, completing the circuit. It is stuffed full of KCl- and other saturated salt solutions. The bridge is necessary for the solution’s ions to move between half-cells, which is necessary for the reaction to proceed. An illustration of a salt bridge is filter paper dip in potassium nitrate or sodium chloride solution.
Types of Electrochemical Cells
There are two main categories of electrochemical cells:
1. Galvanic cells (also known as Voltaic cells)
2. Electrolytic cells
Galvanic Cell
With the aid of a redox reaction, the galvanic cell transforms chemical energy into electrical energy or electricity. Separate compartments are used for the oxidation and reduction processes. An electrolyte solution and a metallic conductor that serves as an electrode are both present in each compartment. Half cells are the compartments where the electrode and electrolyte solution are stored.
Energy releases when electrons change species spontaneously through a redox reaction. When the reaction splits into the two half-reactions of oxidation and reduction, this energy helps to carry out tasks. A wire serves as a bridge between the two containers where these two reactions run, in order to allow electrons to move from one to the other. The electrolyte solutions in a galvanic cell typically contain two different types of metals.
Example of Galvanic Cell
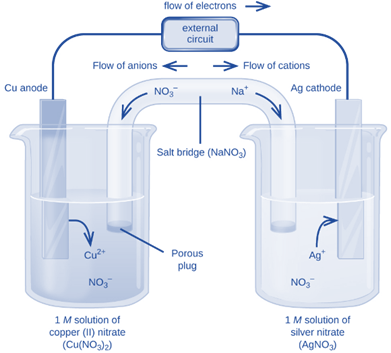
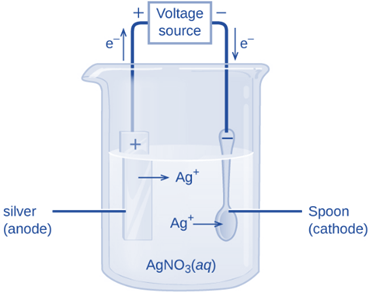
For Example: We can make galvanic/voltaic cells out of solid copper (Cu(s)) in a silver nitrate solution (AgNO3(s)).
AgNO3 disintegrates into Ag+ and NO3– ions during this reaction. The copper electrode will then instantly oxidize Cu(s) to Cu2+(aq) and reduce itself to Ag when it is introduced to this solution of silver ions, Ag+(aq). This reaction must be divided into two separate containers because it will produce energy, as was mentioned above. If not, the energy will waste. The Galvanic/Voltaic cell is then ready after attaching a wire (between the two containers) to allow the floor of electrons between them.
These reactions take place on electrodes, which are metal strips. The term “cathode” refers to the electrode at which reduction occurs or the metal electrode that gains an electron(s), and the term “anode” refers to the electrode at which oxidation occurs or the metal electrode that loses an electron(s).
Cu is the anode and Ag is the cathode in the reaction shown in the preceding example.
The flow of electrons is always from the anode to the cathode.
The cell notation of the above-mentioned galvanic cell is:
Cu(s)│1M Cu (NO3)2(aq)║1M AgNO3(aq)│Ag(s)
Electrolytic cells
These are the electrochemical cells that use electrical energy to initiate a non-spontaneous reaction. These can break down chemical substances like water into hydrogen and oxygen and hence this decomposition is accomplished through a procedure known as electrolysis. Therefore, to perform electrolysis, electrolytic cells require a DC power source, two electrodes, and an electrolyte.
An electrolytic cell converts electrical energy into chemical energy. In this case, an electrolytic solution containing cations and anions is used to immerse the electrodes. When current is supplied, the ions move in the direction of electrodes with opposite polarity, where simultaneous reduction and oxidation occur.
Uses for Electrochemical Cells
- Many non-ferrous metals are electro-refined using electrolytic cells. To electrowine these metals.
- Electrolytic cells help in the production of high-purity lead, zinc, aluminum, and copper.
- By putting molten sodium chloride in an electrolytic cell and running an electric current through it, metallic sodium can be extracted from the solution.
- Galvanic cells are a common component of many commercial batteries, including lead-acid batteries.
- In many remote locations, fuel cells, a significant class of electrochemical cells, also provide clean energy.
References
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_(OpenSTAX)/17%3A_Electrochemistry
- https://www.britannica.com/biography/William-Nicholson-English-chemist-and-inventor
- https://byjus.com/jee/electrochemistry/
- https://byjus.com/chemistry/electrochemical-cell/#:~:text=An%20electrochemical%20cell%20is%20a%20device%20that%20can%20generate%20electrical,electrical%20energy%2C%20or%20vice%20versa.
- https://www.sciencedirect.com/topics/chemistry/electrochemical-cell
