Dysprosium is a chemical element with an atomic number of 66 and is denoted by the symbol ‘Dy’ in the periodic table. It is a soft and bright silvery-white in appearance with metallic luster and is classified as a rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Dysprosium exhibits the propensity to engage in chemical reactions with diverse non-metallic elements under elevated temperatures, resulting in the formation of binary compounds characterized by fluctuating composition and oxidation states of +3, and occasionally +2.
Dysprosium is not naturally occurring in its elemental form but instead exists within various minerals, predominantly monazite, and bastnaesite. The composition of the crust of the earth consists of roughly 5.2 milligrams per kilogram, while the concentration of Dysprosium (Dy) in seawater is approximately 0.9 milligrams per liter.
Interesting Science Videos
History of Dysprosium
- The discovery of dysprosium took place in 1886, courtesy of the efforts of French chemist Paul Émile Lecoq de Boisbaudran. During his research, de Boisbaudran successfully isolated dysprosium oxide from holmium oxide, thus contributing to the identification of the element.
- In 1875, Boisbaudran made the important discovery of gallium, followed by the isolation of samarium in 1879 through the utilization of fractional separation techniques.
- Boisbaudran devised a complex and labor-intensive methodology for the isolation of dysprosium. The testing process consisted of conducting 32 iterations of precipitation of the hydroxide compound through the utilization of ammonia, followed by 26 iterations of precipitation of the insoluble oxalate salt.
- The element was designated as dysprosium, derived from the Greek term ‘dysprositos,’ which translates to ‘difficult to acquire.’
Occurrence of Dysprosium
- The composition of the crust of the earth consists of roughly 5.2 milligrams per kilogram, while the concentration of Dysprosium (Dy) in seawater is approximately 0.9 milligrams per liter.
- Dysprosium is not naturally occurring in its elemental form but instead exists within various minerals, predominantly monazite, and bastnaesite.
- Monazite sand and bastnaesite are the primary sources for commercially recovering it, employing ion exchange and solvent extraction techniques.
- Dysprosium metal is typically obtained through the reduction of its trifluoride compound using calcium metal.
- Dysprosium is an element that exhibits a range of isotopes, specifically 29 of them. These isotopes have been studied and their respective half-lives have been determined. The mass numbers of these dysprosium isotopes span from 141Dy to 169Dy.
Isotopes of Dysprosium
Naturally occurring dysprosium consists of a combination of seven different isotopes: 156Dy, 158Dy, 160Dy, 161Dy, 162Dy, 163Dy, and 164Dy.
Naturally Occurring Isotopes of Dysprosium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 156Dy | 0.06 |
| 158Dy | 0.10 |
| 160Dy | 2.34 |
| 161Dy | 18.91 |
| 162Dy | 25.51 |
| 163Dy | 24.90 |
| 164Dy | 28.18 |
Elemental Properties of Dysprosium
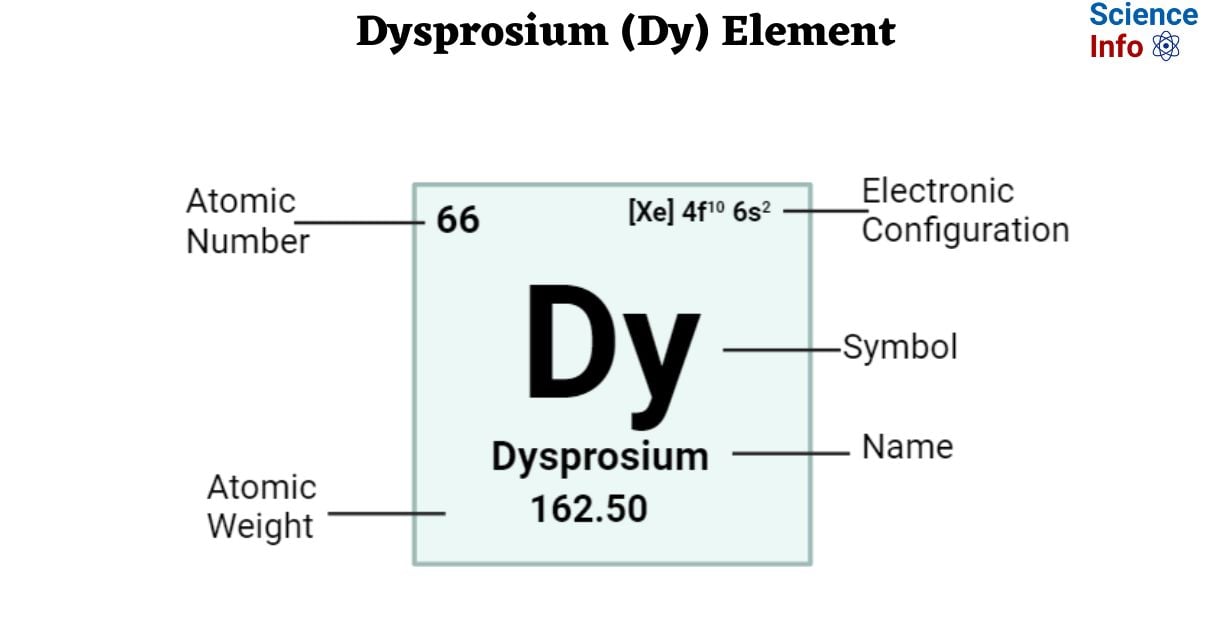
| Electronic Configuration | [Xe] 4f10 6s2 |
| Atomic Number | 66 |
| Atomic Weight | 162.50 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 8.536 g.cm -3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 28, 8, 2 |
| Electrons | 66 |
| Protons | 66 |
| Neutrons in the most abundant isotope | 98 |
Physical Properties of Dysprosium
- Dysprosium has an atomic number of 66 and is a silvery-white rare earth metal. It has a melting point of 1407°C (2565 °F) and a boiling point of 2562 °C (4653 °F).
- Dy has a solid phase density of 8.540 g/cm3 and a liquid or molten phase density of 8.37 g/cm3.
- Dysprosium exhibits a vibrant silver metallic luster.
- The physical properties of Dy can be significantly influenced by even minute quantities of impurities.
- Dysprosium is soft meaning it can be easily cut into pieces with a knife.
- Dysprosium exhibits a simple ferromagnetic order when its temperature is below 85K (-188.15°C).
- The system undergoes a transition to helical antiferromagnetism within the temperature range of 85 to 179K.
- Dysprosium exhibits disordered antiferromagnetic ordering when the temperature exceeds 179K (-94.15°C).
- Dysprosium, a chemical element, exhibits malleability, indicating its ability to be easily shaped into thin sheets without any tendency to break along specific planes.
- Dysprosium (Dy) exhibits ductility, a property that allows it to be drawn into thin wires without fracturing.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1680 K (1407 °C, 2565 °F) |
| Boiling point | 2840 K (2562 °C, 4653 °F) |
| Density | 8.540 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.22 (Pauling Scale) |
Chemical Properties of Dysprosium
- Dysprosium exhibits a relatively low reactivity when exposed to room temperatures. The substance exhibits a relatively slow rate of oxidation upon exposure to atmospheric conditions.
- Dysprosium exhibits a notable property of maintaining its luster when exposed to dry air, but it undergoes rapid tarnishing when exposed to moist air.
- Dysprosium exhibits a high reactivity when exposed to air, undergoing combustion to produce dysprosium (III) oxide.
- Dy metal exhibits a chemical reaction with halogens at temperatures exceeding 200°C, resulting in the formation of the respective trihalides.
- Dysprosium exhibits the propensity to form binary compounds with a range of non-metals, including nitrogen, phosphorus, carbon, sulfur, and barium, under conditions of elevated temperatures.
- Dy exhibits a notable degree of electropositivity, as evidenced by its characteristic reactivity. It undergoes a gradual reaction with cold water and a more rapid reaction with hot water, ultimately resulting in the formation of dysprosium hydroxide.
Chemical Reactions of Dysprosium
- The Reaction of Dysprosium With Water
Dysprosium exhibits a sluggish reaction rate when exposed to cold water, whereas it undergoes a relatively rapid reaction when in contact with hot water. This reaction results in the formation of dysprosium hydroxide (Dy(OH)3) and the liberation of hydrogen gas (H2).
2 Dy (s) + 6 H2O (g) → 2 Dy(OH)3 (aq) + 3 H2 (g)
- The Reaction of Dysprosium With Air
The dysprosium metal exhibits a gradual oxidation process when exposed to atmospheric conditions, resulting in the formation of dysprosium (III) oxide, denoted as Dy2O3, which can be readily ignited.
4 Dy (s) + 3 O2 (g) → 2 Dy2O3 (s)
[when heated Δ]
- The Reaction of Dysprosium With Halogens
Dysprosium metal exhibits reactivity with various halogens, resulting in the formation of dysprosium (III) halides.
The chemical reaction between dysprosium metal and fluorine gas (F2) results in the formation of dysprosium (III) fluoride (DyF3).
2 Dy (s) + 3 F2 (g) → 2 DyF3 (s) [green]
The chemical reaction between dysprosium metal and chlorine, represented by (Cl2) results in the formation of dysprosium (III) chloride, denoted as (DyCl3).
2 Dy (s) + 3 Cl2 (g) → 2 DyCl3 (s) [white]
The chemical reaction between dysprosium metal and bromine, represented by (Br2) results in the formation of dysprosium (III) bromide, denoted as (DyBr3).
2 Dy (s) + 3 Br2 (g) → 2 DyBr3 (s) [white]
The chemical reaction between dysprosium metal and iodine (I2) results in the formation of dysprosium (III) iodide, denoted as (DyI3).
2 Dy (s) + 3 I2 (g) → 2 DyI3 (s) [green]
- The Reaction of Dysprosium With Acid
Dysprosium metal exhibits a high degree of solubility when subjected to dilute sulfuric acid, which results in the formation of solutions that includes the yellow aquated Dy (III) ion, alongside the liberation of hydrogen gas, H2. The existence of Dy3+ (aq) as predominantly the complex ion [Dy(OH2)9]3+ is highly probable.
2 Dy (s) + 3 H2SO4 (aq) → 2 Dy3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Uses of Dysprosium
Dy is used in various industries some of which are discussed below:
Used In Alloys
Dysprosium is a crucial element utilized in the production of magnetostrictive alloys. Dysprosium is commonly utilized in the production of alloys that are essential components in a wide range of electrical and electronic devices. An alloy is created through the process of melting and combining two or more mether. The mixture exhibits distinct properties that are not found in any of ial elements. Certain dysprosium alloys possess exceptional magnetic properties, rendering them highly valuable in the context of CD players.
Used In Magnets
Magnetostriction refers to the intriguing property exhibited by magnetic materials, wherein the change in shape or dimension when subjected to magnetization. Terfenol-D is an alloy composed of terbium, iron, and dysprosium. It exhibits the property of magnetostriction, meaning it can expand or contract when subjected to a magnetic field. This unique characteristic makes it valuable for various applications such as ships’ sonar systems, sensors, and transducers. Terfenol-D is utilized in a speaker known as the ‘SoundBug’, which has the ability to transform any flat surface into a functional speaker.
Used In Lasers and Lights
Dysprosium is an essential component used in the production of lasers and various lighting materials. Dysprosium compounds, such as dysprosium halide, are commonly used in commercial lighting applications, specifically in halide discharge lamps. These compounds play a crucial role in generating a bright and vibrant white light. Additionally, it finds application in the film industry for medium-source rare-earth lamps (MSRs). Dysprosium iodide is utilized in these lamps for the purpose of generating a highly vibrant white light.
Used In Nuclear Reactors
A mixture of nickel along with dysprosium oxide is employed in the production of control rods for nuclear reactors. These control rods have been engineered to efficiently soak up neutrons over an extended period of time, without experiencing any contraction or expansion. Dysprosium exhibits a strong affinity for absorbing neutrons, which are minute particles found within atoms and are generated during nuclear reactions. Dysprosium-containing metal rods, known as control rods, are utilized in nuclear reactors to regulate the availability of neutrons and control their rate.
Miscellaneous Uses
- The nanofibers composed of dysprosium compounds exhibit notable mechanical strength and possess a significantly expansive surface area. Hence, they possess the capability to strengthen additional substances and serve as catalysts.
- Dysprosium gallium garnet (DDG), dysprosium aluminum garnet (DAG), and dysprosium iron garnet (DyIG) find application in the realm of adiabatic demagnetization refrigeration.
- Dysprosium is capable of detecting external radiation intake. Dysprosium-doped calcium sulfate or fluoride crystals are used. The crystals contain dysprosium atoms that emit radiation, causing them to shine. Luminescence is used to measure exposure in dosimeters.
Health Effects of Dysprosium
- Dysprosium does not possess any biological function.
- Dysprosium salts that are dissolved pose a mild toxicity risk if ingested, whereas insoluble salts are considered non-toxic.
- Toxicity tests conducted on mice determined that a minimum dose of 500 grams is required to pose a life-threatening risk to humans.
Environmental Effects of Dysprosium
There is no evidence that dysprosium is harmful to either plants, animals, or the environment.
Video on Dysprosium Element
References
- Emsley, John (2011). Nature’s Building Blocks: An A–Z Guide to the Elements (2nd ed.). Oxford: Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Oxford: Butterworth Heinemann. ISBN 0-7506-3365-4.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1-4398-5511-0.
- https://byjus.com/chemistry/dysprosium/
- https://www.chemicool.com/elements/cerium.html
- https://www.lenntech.com/periodic/elements/dy

