A chemical reaction occurs when a chemical change occurs. In other words, a chemical reaction causes a chemical change in the reactants. There are several types of chemical reactions. All potential chemical reactions are classified into many groups based on their nature. One of them is the double displacement reaction.
A double displacement reaction occurs when two ionized compounds exchange ions, resulting in the formation of two new compounds.
Interesting Science Videos
What is Double Displacement Reaction?
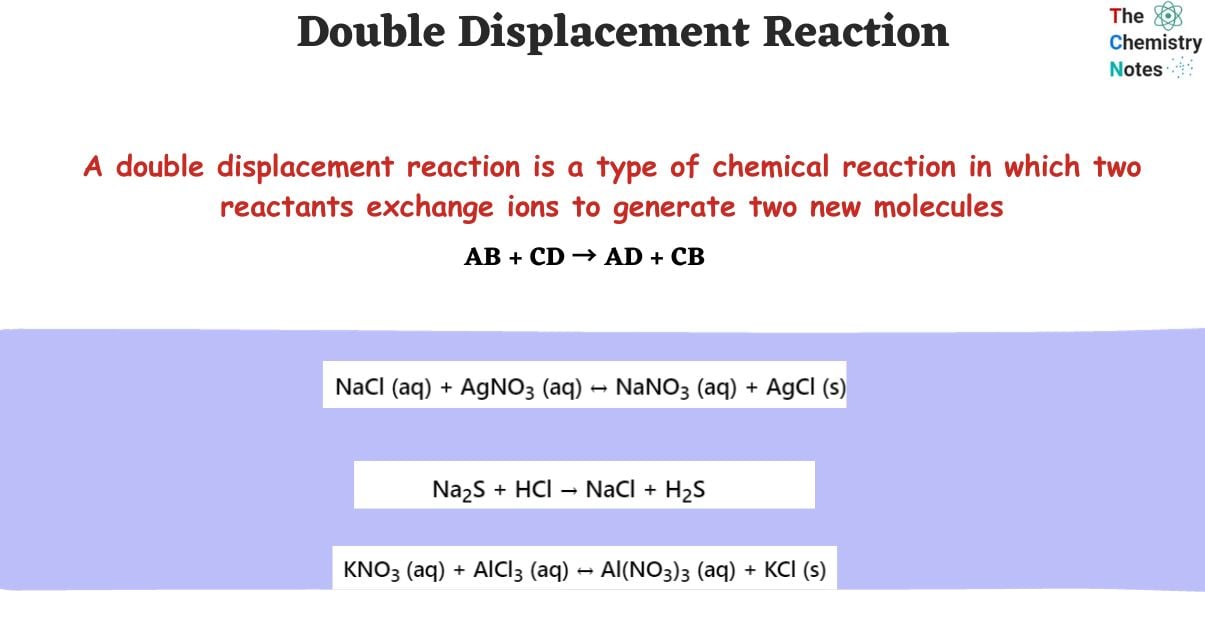
A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. Acids and bases, as well as ionic substances, generally engage in a double-displacement process. The bonds that form in the reactant molecules and the result compounds are the same kind of bonds.
Double displacement reactions give the form:
AB + CD → AD + CB
The positive-charged cations and negative-charged anions of the reactants exchange places (double displacement) to generate two new products in this sort of reaction.
A+B– + C+D– → A+D– + C+B–
A double displacement reaction is also known as a metathesis reaction or a double replacement reaction.
Different Types of Double Displacement Reactions
Neutralization, alkylation, acid-carbonate reactions, counter-ion exchange, aqueous metathesis with precipitation (precipitation processes), and aqueous metathesis with double decomposition (double decomposition reactions) are all examples of double replacement reactions. However, in general chemistry, the two most common kinds are neutralization reactions and precipitation reactions.
Neutralization Reaction
A neutralization reaction involves the double displacement of acids and bases. When water is used as the silver, the reaction normally results in the formation of an ionic compound—a salt. If one or both of the reactants are strong acids or bases, the reaction will go forward.
A neutralization reaction is the reaction of hydrofluoric acid and sodium hydroxide in water to generate water and sodium fluoride. Hydrofluoric acid is an acid (naturally), whereas sodium hydroxide is a basic. The reaction has the following broad form:
acid + base → water + salt
The reaction in this example is:
HF (aq) + NaOH (aq) → H2O + NaF (aq)
Another example of a neutralization reaction is the chemical reaction between baking soda and vinegar in the baking soda volcano. The reaction eventually creates a gas (carbon dioxide) and a salt (sodium carbonate), although the first neutralizing process produces carbonic acid (H2CO3) and sodium acetate (NaCH3COO).
NaHCO3 + CH3COOH(aq) → H2CO3 + NaCH3COO
Because of the way the compound formulae are stated, it is more difficult to see that the cations are exchanging anions. When the atoms in the anions of the reactants and products are compared, the reaction is identified as double replacement.
Precipitation Reaction
In a precipitation process, two aqueous ionic compounds combine to generate an insoluble ionic product. One example is the interaction between lead(II) nitrate and potassium iodide, which produces potassium nitrate and (insoluble) lead iodide.
Pb(NO3)2 (aq) + 2 KI (aq) → 2 KNO3 (aq) + PbI2(s)
The precipitate is the name given to the insoluble product. The solvent and soluble components of the reaction are referred to as the supernatant or supernate. We may utilize solubility criteria to anticipate if a precipitation reaction will occur. The catalyst that propels the reaction in that direction is the development of a solid precipitate.
Silver chloride (AgCl) precipitates in sodium nitrate solution (NaNO3) as a result of the interaction between silver nitrate (AgNO3) and sodium chloride (NaCl).
NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s/ppt)
When barium chloride (BaCl2) reacts with sodium sulfate (Na2SO4), sodium chloride (NaCl) and a white precipitate of barium sulfate (BaSO4) are formed.
BaCl2 (aq) + Na2SO4 (aq) → NaCl (aq) + BaSO4 (s/ppt)
Gas Formation
A gas formation process produces a gas as a product. The gaseous product rises up from the solution and escapes into the atmosphere
When sodium sulfide (Na2S) and hydrochloric acid (HCl) solutions are combined, the reaction produces aqueous sodium chloride (NaCl) and hydrogen sulfide (H2S) gas.
Na2S (aq) + 2 HCl (aq) → 2 NaCl (aq) + H2S (g)
Aqueous zinc chloride (ZnCl2) and hydrogen sulfide (H2S) gas are produced when zinc sulfide (ZnS) is dissolved in hydrochloric acid (HCl).
ZnS (s) + 2 HCl (aq) → ZnCl2 (aq) + H2S (g)
Examples of Double Displacement Reactions
- Aluminum nitrate (Al(NO3)3) and potassium chloride (KCl) are formed when potassium nitrate (KNO3) interacts with aluminum chloride (AlCl3).
KNO3 (aq) + AlCl3 (aq) ↔️ Al(NO3)3 (aq) + KCl (s)
- Sodium chloride (NaCl) and silver nitrate (AgNO3) react to form sodium nitrate (NaNO3) and silver chloride (AgCl).
NaCl (aq) + AgNO3 (aq) ↔️ NaNO3 (aq) + AgCl (s)
The silver exchanged its nitrite ion for sodium’s chloride ion.
- Another example is the reaction of sodium sulfide with hydrochloric acid, which results in the formation of sodium chloride and hydrogen sulfide:
Na2S + HCl → NaCl + H2S
How to identify a Double Replacement Reaction?
In a chemical equation, a double replacement reaction can be identified:
- By observing if the cations exchange anions with each other.
- Look for a reaction between two aqueous solutions that gives one aqueous product (aq) and one that precipitates to create a solid product (s) if the states of matter of the reactants and products are provided.
- If you don’t know the reactants but see precipitate development after combining them, a double replacement reaction is likely.
- If you can’t see the reaction, you can use solubility criteria to estimate whether the reactants will dissolve and a precipitate will develop (indicating a double replacement reaction).
Frequently Asked Questions (FAQ)
Why does Double-replacement Reaction Occur?
When two ionic chemicals react, a double-replacement reaction occurs. The positive ions (cation) and negative ions (anion) of the two ionic molecules, which are reactants, swap positions. The reaction produces two new products, both of which are ionic compounds.
What is the difference between single- and double-replacement reactions?
A more reactive element replaces a less reactive element from a molecule in a single-replacement reaction. In a double-replacement process, two or more atoms exchange positions to generate new molecules.
Is it possible for a double displacement reaction to be a redox reaction?
No. A redox reaction requires components to change oxidation states, which does not occur in a double displacement reaction.
How to Identify a Double-replacement Reaction?
The simplest technique to recognize a double displacement reaction is to determine if the cations swapped anions with each other. If the states of matter are mentioned, check for watery reactants and the development of a single solid product, as the reaction often produces a precipitate.
Video on Double Displacement Reaction
References
- https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/types-of-chemical-reactions/a/double-replacement-reactions
- Helmenstine, Anne Marie, Ph.D. “Double Replacement Reaction Definition.” ThoughtCo, Apr. 5, 2023, thoughtco.com/definition-of-double-replacement-reaction-605046.
- https://sciencenotes.org/double-replacement-reaction-definition-and-examples/
- https://unacademy.com/content/question-answer/chemistry/what-is-a-double-displacement-reaction/
- https://sciencing.com/what-is-a-double-replacement-reaction-13710476.html


Learning this in school.