Spectrophotometers are instruments that measure the distribution of light through different wavelengths. Scientists utilize these devices to measure various types of light, such as visible and near-ultraviolet light. In order to obtain measurements at a faster speed and with greater precision, scientists have found that the double-beam spectrophotometer is a suitable solution.
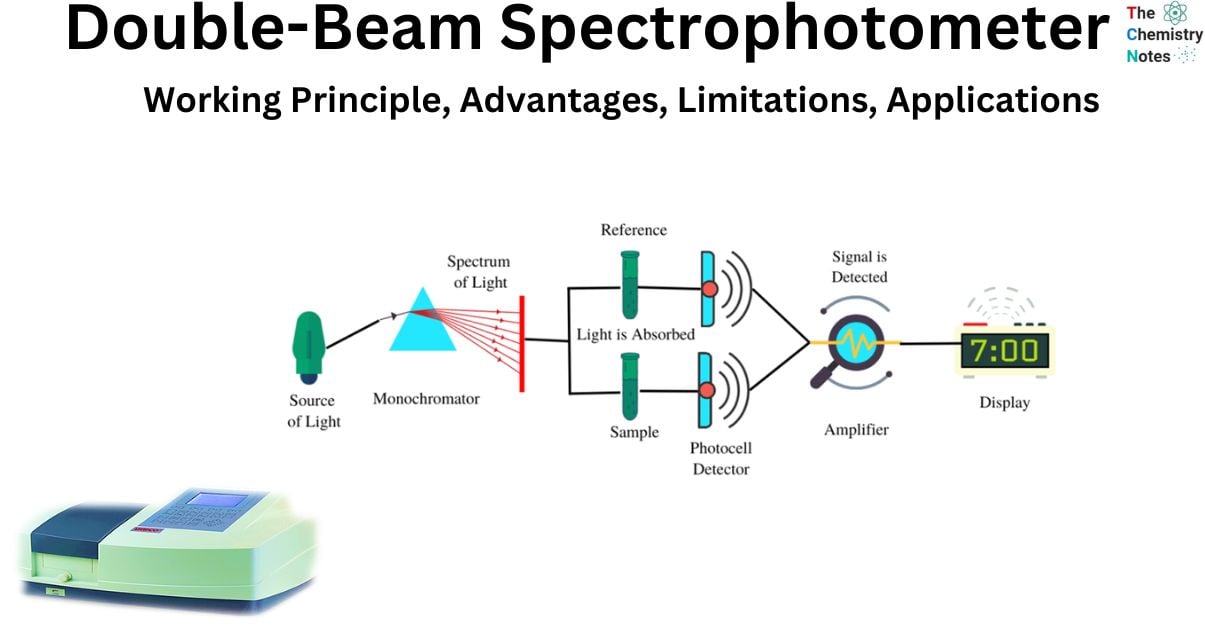
A double-beam spectrophotometer is an analytical tool utilized for measuring the light absorption of liquid or gas samples contained in graduated cylinders. Transmission in a double-beam spectrophotometer is often accomplished by the utilization of ultraviolet radiation, infrared rays, and visible light. However, the more current and modern spectrophotometers make use of a broad spectrum of electromagnetic wavelengths in order to perform the reflection and transmission processes. This spectrum of electromagnetic wavelengths includes visible rays, infrared rays, microwave rays, and ultraviolet rays.

The determination of the amount of light that corresponds to a given wavelength is the primary function of a double-beam spectrophotometer. In this stage of the process, the analyte present in the biological sample absorbs the beams of light. In spectrophotometry, the specimens, also known as samples, can be either gases or liquids. During the process of spectrophotometry, the analytes are mixed with a solvent and then allowed to dissolve. In order to prepare the solid pellets for usage as an analyte in spectrophotometry, the solvents that are currently being utilized are first combined with a transparent matrix. When making the disks for the double-beam spectrophotometer, a pellet press is used. This press has a disk that is suspended in the biological samples, and the light beams pass through this disk as they move through the press.
What is a double-beam spectrophotometer?
The double-beam spectrophotometer is a common practice for determining the transmittance or reflectance of analytes present in a given sample. The specimens utilized in this study are either solid materials that possess transparency or opacity, such as polished glass, or gaseous substances that exhibit solvent properties. It has been observed that numerous biochemical compounds possess inherent pigmentation, resulting in their capacity to absorb either visible or ultraviolet radiation. Within the spectrophotometer, colorimetric processes can be used to measure the amount of light that has been transmitted. Even the colorless biochemicals that are used in this procedure have the potential to be transformed into colourful compounds. It is appropriate for use in chromogenic color-forming processes, which can then lead to the formation of compounds that are appropriate for colorimetric analysis using a single or double-beam spectrophotometer.
It has been observed that they can also be engineered to assess the diffusivity of numerous analytes. This phenomenon is not limited to any specific light range and may occur across the various spectral wavelengths utilized by researchers in their biological investigations. In spectrophotometry, the typical wavelength range covered by light rays is between 200 nm and 2500 nm, which can be achieved through various controls and calibrations. Calibrations are required for the instrument within the specified ranges of light beams, utilizing standards that vary in type based on the wavelengths of light involved in the photometric determination.
Why double-beam spectrophotometer is called so?
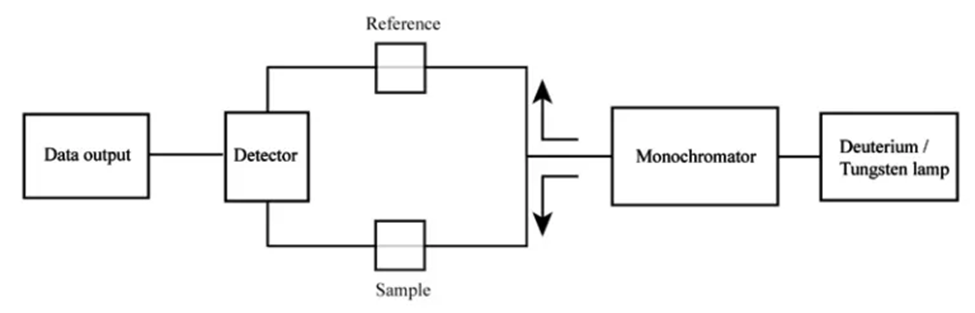
The device is referred to as a “double-beam” spectrophotometer due to its utilization of two light beams:
- Reference beam: The beam is transmitted through the reference standard for the purpose of monitoring the energy of the lamp.
- Sample beam: The aforementioned beam traverses the sample medium in order to reflect the absorption of said sample.
Double-beam spectrophotometer (Source: https://www.scientificsarkar.com/double-beam-spectrophotometer/)
The diagrams of a double-beam spectrophotometer illustrate the manner in which the mechanical chopper partitions the energy emanating from the light source by means of a half mirror. This results in the segregation of one beam to the reference side and the other beam to the sample side. The utilization of this particular format enables the reference and the sample to be concurrently perused, thereby facilitating a prompt point of reference. The utilization of an infrared thermometer and photometers in a dual-beam spectrophotometer enables the measurement of absorbance in relation to wavelength, thereby facilitating the detection of the color of the sample. The determination of absorbance involves the calculation of the ratio between the beams of the sample and the reference.
In simple words, prior to their arrival at a monochromator unit that is linked to the optical apparatus, the light beams may undergo recombination subsequent to excitation. Spectrophotometry utilizes a pair of monochromators in specific scenarios. The fundamental principles underlying a standard double-beam spectrophotometer are the dispersion, reflection, and refraction of light. By employing these methodologies, it is possible to compute the results of analytes that exist in a biological specimen by relying on their absorption characteristics.
Working principle of double-beam spectrophotometer
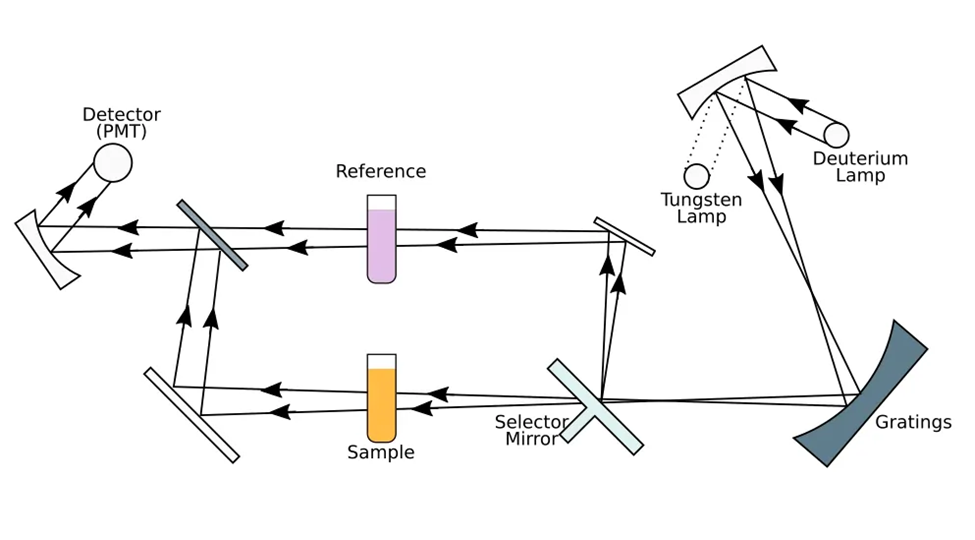
The fundamental working principle of a double-beam spectrophotometer is predicated upon the phenomena of light ray reflection and transmittance. The primary component of both single and double-beam spectrophotometers is the light source, which emits intense beams of light. The characteristics of these light beams are reliant upon the specific wavelength of interest, which may include electrically powered ultraviolet and visible rays, as well as infrared rays.
- The monochromator effectively isolates light beams of a particular analytical wavelength from the broader spectrum of the light source. The selection of wavelengths is based on the spectral composition of the light rays that traverse the double-beam spectrophotometer. The wavelength of the light ray employed in an instrument is determined by the absorbance properties of the analyte under consideration.
- Instruments known as monochromators have as their primary function the facilitation of the passage of polychromatic light beams via the entry slit of the monochromator instrument. It also makes it possible for a single light ray wavelength that is monochromatic to depart the device via the exit slit.
- This fascinating and extremely focused beam of light is made up of a relatively small portion of the electromagnetic spectrum of light.
- The slit methods or apertures of the monochromator that is attached to the spectrophotometer are what determine the spread of the wavelengths as well as the electromagnetic band-pass of those wavelengths.
- The light beams in the single and double-beam spectrophotometer can be manipulated with the help of these monochromators, which include customizable settings. In this case, the process of transmission and reflection is aided by the characteristic of the light rays, which is what disperses the elements in the monochromator.
The light source in a double-beam spectrophotometer is utilized in the instrumental design to irradiate the samples with light beams. During the process of spectrometry, the light rays undergo a diversion of 90 degrees. The process involves the rotation of a disk containing three distinct panels within the spectrophotometers. One of the components of the apparatus facilitates the unobstructed transmission of the light beam through the disk without any incidence. And then another point consists of a mirror surface, with a third point which is black. The direct illumination of the specimen cells occurs when the light beam traverses the disk panels. In the event that the sample under consideration is in a liquid or gaseous state, it is housed within a cuvette that is composed of a transparent material which exhibits no absorption of light rays within the spectral range relevant to the double-beam spectrophotometer.
The substance of interest present in the specimen is solubilized in a solvent that is contained in the cuvette component of the dual-beam spectrophotometer. When the incident light rays are reflected at a 90-degree angle by the rotating disk, they do not come into contact with the biological specimen. The incident light traverses a cuvette segment within the reference cell that exclusively comprises the solvent. These phenomena facilitate the propagation of electromagnetic radiation and the phenomenon of specular reflection.
- In the third sequence of light rays in the spectrophotometer, the black sector of the disk obstructs the light source beam, resulting in no transmission of light to the sample. Consequently, the photo transducer in the instrument does not receive any light rays.
- The computer employs this phase of the light ray cycle to quantify and gauge the dark current. The term “dark current” refers to the quantity of light generated by the photo transducer circuitry within a dual-beam spectrophotometer. This phenomenon occurs when there is an absence of incident light rays on the photo transducer component. It is possible to mitigate the effects of dark current by utilizing the aggregate light measurements obtained through the use of a spectrophotometer.
In a spectrophotometer, the light rays that do not get absorbed after traveling through every portion of the specimen cell or sample cell are directed onto the photo transducer or light detector. This occurs after the light rays have traveled through every part of the specimen cell or sample cell. The arrival of photons in the light beams is translated into an electrical signal by this component’s particular portion of the part. This conversion is carried out by using the screen of the computer. The spectrophotometer can be configured in such a way that it does not require the light pathways to be in a straight line. It’s also possible for it to be a mess. As can be observed, the light beam of rays within the apparatus is able to be redirected through the use of mirrors, and it is also able to be scattered with the assistance of collimation.
Difference between single beam and double-beam spectrophotometer
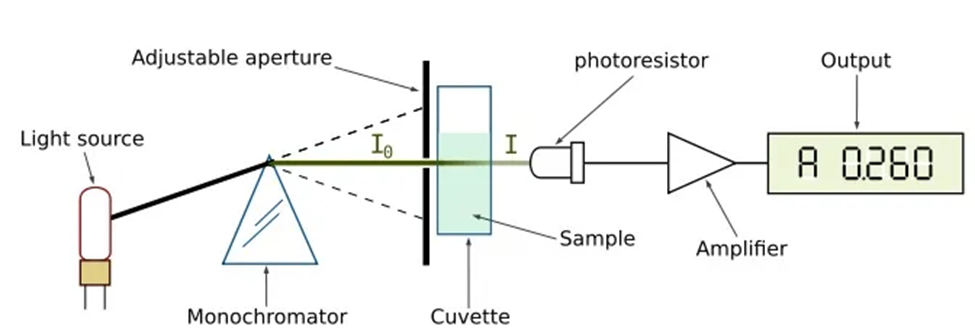
One type of spectrophotometer, known as a single beam spectrophotometer, is an analytical device in which all of the light waves emanating from the light source pass through the sample. Because of this, the intensity of the light both before and after the light passes through the sample is what is measured. These single beam spectrophotometers have a smaller footprint and a more straightforward optical design compared to their double-beam counterparts. Additionally, the cost of these instruments is more reasonable. Since it utilizes a single light beam that is not split in any way, the sensitivity of the detection of the light beam after it has passed through the sample is very great. As a result, high energy exists throughout. Single beam spectrophotometers are available for use in analysis at wavelength ranges ranging from visible to ultraviolet.
A single beam spectrophotometer is a type of spectrometer that determines the concentration of an analyte in a sample by determining the amount of light that is absorbed by the analyte in question. This is the point at which the Beer-Lambert Law kicks into effect. According to this equation, the concentration of an analyte is directly proportional to the absorbance of the substance.
An analytical equipment known as a double-beam spectrophotometer divides the light beam that is originating from the light source into two distinct portions. While one portion serves as a standard (the beam used for the reference), the other portion is used to analyze the sample (the beam used for the sample). As a direct consequence of this, the reference beam is unable to travel through the specimen. The beam specimen has the capability to quantify the degree of light absorption exhibited by the sample. The absorption of a sample can be measured by comparing it with a reference beam.
Hence, the absorption can be defined as the quotient of the sample beam, which has traversed the sample, and a reference beam. The spectrophotometer employs a monochromator to selectively isolate the desired wavelengths from a light beam. Upon recombination, the reference beam and sample beam proceed toward the monochromator. As a result, this approach mitigates or counteracts the electronic and mechanical perturbations on both the sample and reference beams in an equitable manner.
The basic difference between single beam and double-beam spectrophotometers lies in their respective optical configurations. Specifically, in single beam spectrophotometry, the sample is exposed to the entirety of the incident light beam, whereas in double-beam spectrophotometry, the incident light beam is bifurcated into two separate beams, with only one of these beams passing through the sample. Spectrophotometers are scientific devices utilized for the purpose of measuring the concentration of analytes present in a given sample through the application of a light beam. Consequently, this methodology quantifies the light absorption of the specimen.
Advantages of double-beam spectrophotometer
- More consistent detection: Recent advancements in optics have facilitated a significant degree of automation and provide comparable, if not superior, levels of detection in contrast to previous single beam systems. The real-time measurement is not affected by instability factors such as lamp drift, stray light, and voltage fluctuations.
- Minimal or negligible warm-up time for the lamp is necessary. This practice not only enhances the rate of output but also preserves the longevity of the lamp.
- The utilization of a double-beam spectrophotometer offers significant advantages, notably the quick and consistent acquisition of measurement.
- It is not imperative to modify the transmittance within the range of 0% to 100% for every wavelength.
- Simultaneously, it exhibits the ratio of intensity between the beams of the sample and the reference.
- Efforts are made to minimize fluctuations in the radiation source.
- ● This technology enhances the efficiency of scanning over a wide range of wavelengths.
Limitations of double-beam spectrophotometer
- It is quite expensive
- The use of a double-beam spectrophotometer presents a considerable challenge.
Application of double-beam spectrophotometer
The utilization of a double-beam spectrophotometer, as opposed to a single beam spectrophotometer, can prove advantageous for applications that necessitate stability, flexibility, and speed. The aforementioned tools are utilized within both research and clinical laboratory settings for the purpose of:
- Wavelength scanning
- Protein analysis
- DNA analysis
- Quantitative analysis
- Kinetics study
Double-beam spectrophotometers are utilized by physicists, biologists, and chemists to quantify visible, near-infrared, and near-ultraviolet radiation.
Summary of double-beam spectrophotometer
The double-beam UV-Visible spectrophotometer is a scientific tool utilized for the purpose of quantifying the light absorption of a given sample. The working principle entails partitioning the light output emanating from a monochromator into two distinct beams, namely the reference beam and the sample reading beam. The reference beam is utilized for the purpose of measuring the energy of the lamp, whereas the sample beam is employed to reflect the absorption of the sample. The recombination of the two beams occurs prior to their arrival at a monochromator, which enables researchers to determine the existence of analytes within a biological specimen by evaluating their absorption characteristics. The apparatus employs the principles of light dispersion, reflection, and refraction to precisely gauge the absorption properties. The utilization of double-beam spectrophotometer is feasible for the analysis of organic compounds through the assessment of their radiation absorption capacity.
In short, The double-beam spectrophotometer facilitates the transmission of light rays that result from the reflection process. The utilization of a single-beam spectrophotometer is analogous to that of a double-beam spectrophotometer. The biological specimens are illuminated by a light source, and subsequently, the results are digitized through a computer system.
References
- Spetrophotometer-en’By GYassineMrabetTalk
- https://www.scientificsarkar.com/double-beam-spectrophotometer/
- https://www.shsu.edu/~chm_tgc/primers/spect.html
- https://www.hunterlab.com/blog/application-of-double-beam-spectrophotometer/
- https://www.smacgigworld.com/blog/double-beam-spectrophotometer.php
- https://www.differencebetween.com/difference-between-single-beam-and-double-beam-spectrophotometer/

The difference between single & double beam methods is not clearly established…
For Educational purpose