Interesting Science Videos
Electronegativity
Electronegativity (E.N) is the general tendency of an atom in a molecule to draw the shared pair of electrons toward itself.
It is defined as an atom’s ability to attract electrons to itself in a chemical bond. It is the chemical variable that determines the kind of bonds that are formed between atoms.
An atom’s ability to attract the electrons of a bond increases with electronegativity, a property of an atom. The electrons in a covalent bond are shared equally by two bonded atoms if their E.N values are the same. Typically, the more electronegative atom attracts the electrons in a chemical bond more so than the other atom. Consequently, a polar covalent bond is created. The electrons aren’t shared at all if the E.N values are very dissimilar. Ionic bonds are created when one atom essentially steals the other atom’s bond electrons.
Many definitions of E.N exist, and they can be roughly categorized as either spectroscopic (defined for isolated atoms) or thermo-chemical (characterizing bond energies and heats of formation of compounds).
Jons Jacob Berzelius, a physicist, coined the term “electronegativity” in 1811. Linus Pauling found the property of electronegativity completely in the year 1932, after numerous discoveries and discussions, and he also constructed an electronegative scale that depends on the bond enthalpy. This has also aided in the development of the valence bond hypothesis.
Electron Affinity
Electron affinity is the energy released when an electron is added to a neutral, gaseous, and isolated atom in its ground state to form a monovalent anion.
It is a quantitative measurement of the energy change caused by adding a new electron to a neutral atom or molecule in the gaseous state. So, electron affinity is a measure of how strongly an incoming electron attracts the nucleus. As a result, the greater the attraction, the more energy is released. The higher an atom’s affinity for electrons, the higher will be its electron affinity value. It is denoted by the symbol Ea and is usually expressed in kJ/mol units.
The reaction that occurs when an atom accepts an electron can be expressed as follows:
X + e− → X− + energyAdditionally, electron affinity can be defined as the amount of energy required to remove an electron from a single negatively charged ion:
X− + energy → X + e−However, electron affinities are much smaller than ionization energies because removing electrons from an anion is easier than removing electrons from a neutral atom. Electron affinities are more difficult to measure and usually less accurate than ionization energies. Fluorine and oxygen have large and negative electron affinities, whereas metals have small and positive affinities.
Robert S Mullikan created the electronegativity scale by combining a number of elements’ electron affinities. The theory of electron affinity is also used in the development of concepts for chemical hardness and chemical potential.
Difference Between Electronegativity and Electron Affinity
- Electronegativity and electron affinity are two separate chemical characteristics associated with elements.
- The major distinction between electronegativity and electron affinity is that electronegativity is the attribute associated to an electron’s capacity to attract towards an atom.
- Electron affinity, on the other hand, is associated with the release of energy whenever an electron seeks to be added to an atom.
- These two traits are frequently associated, although they are not interchangeable. Both electronegativity and electron affinity are concerned with electron transport.
- However, electronegativity is a property of attraction, whereas electron affinity is a property of energy change.
7 Major Difference Between Electronegativity and Electron affinity
Some of the major difference between electronegativity and electron affinity are given in the table below:
| Differences | Electron Affinity | Electronegativity |
|---|---|---|
| Definition | Electron affinity is the energy released when an electron is added to a neutral, gaseous, and isolated atom in its ground state to form a monovalent anion. | It is defined as an atom’s ability to attract electrons to itself in a chemical bond. |
| Measurement Unit | It is measured in kJ/mol or eV. | It is measured in Pauling scale. |
| Affecting Factors | Electronic configuration, atomic size, and nuclear charge of the atoms are the factors that affect electron affinity. | Difference between the valence electrons, atomic number, and the charged nucleus are the factors affecting electronegativity. |
| Associating Atom | It is applied to either atom or a molecule. | It is applied to a single atom. |
| Property | It is quantitative. | It is qualitative. |
| Maximum Value | When there is a strong attracting energy, the maximum value is attained. | When the attracting energy is high, the maximum value is obtained. |
| Examples | Chlorine has the highest electron affinity, while neon has the lowest. | Fluorine is the most electronegative element, while francium is the least electronegative. |
Trend in Electronegativity and Electron affinity in Periodic Table
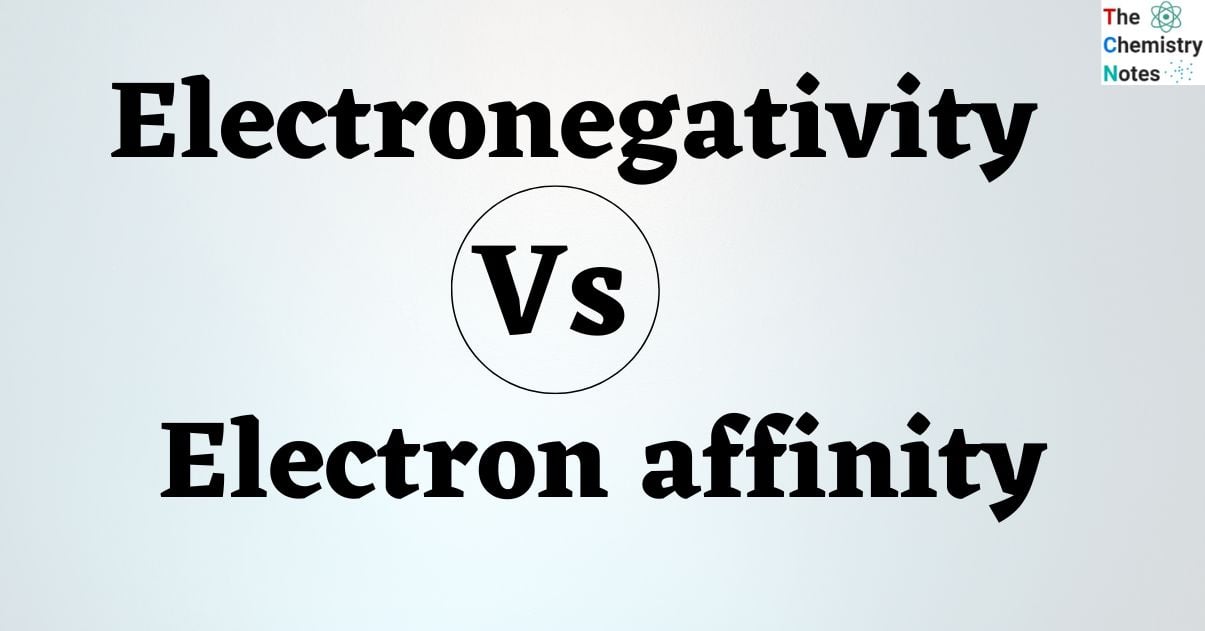
Electronegativity rises from left to right across the periodic table. This is due to a increased number of protons as the atomic number increases. Electronegativity falls from top to bottom as atom size increases. As a result, fluorine is considered the most electronegative element, whereas francium and cesium is considered the least electronegative element. Halogens have a high electronegativity, whereas alkali metals and alkaline earth metals have a low electronegativity.
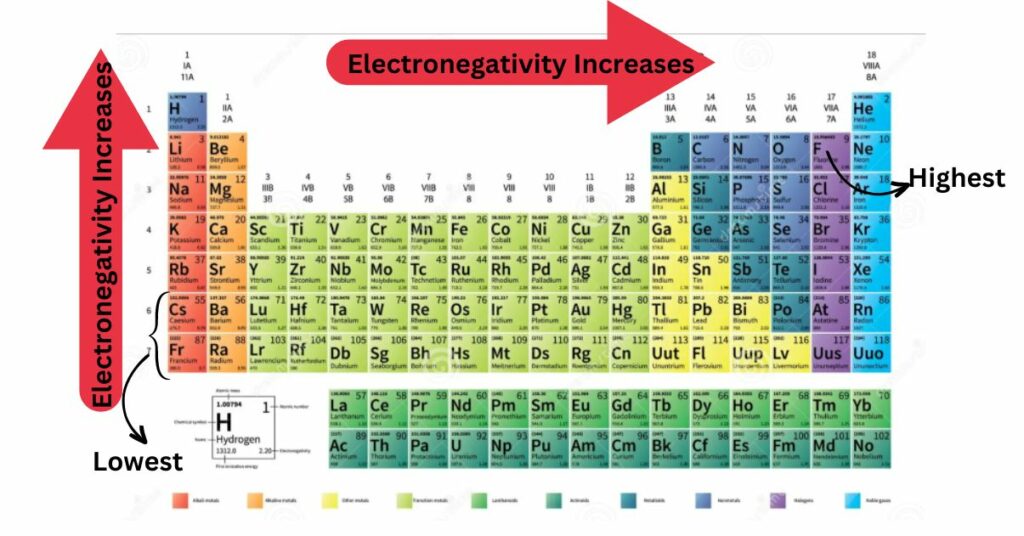
Electron affinity, like ionization energy, electronegativity, atomic or ionic radius, and metallic character, follows periodic table trends. Unlike some of these other characteristics, there are numerous exceptions to the trends for electron affinity.
- Electron affinity increases in general as you move across a row or period of the periodic table, until you reach group 18 or the noble gases. This is due to the filling of the valence electron shell as it moves through a period. For example, a group 17 (halogen) atom gains an electron, whereas a group 1 (alkali metal) atom needs add multiple electrons to reach a stable valence shell. Furthermore, the effective nuclear charge increases as you move across a period.
- The electron affinities of noble gases are low.
- Nonmetals, with a few exceptions, have a greater or more positive electron affinity value than metals.
- Atoms that generate more stable anions than neutral atoms have high electron affinity values.
- Although it is commonly portrayed on a periodic table trend diagram, electron affinity does not consistently decrease as one moves down a column or group. Electron affinity really increases as you proceed down the periodic table in group 2 (alkaline earth metals).
Video on Difference Between Electronegativity and Electron affinity
References
- https://www.vedantu.com/chemistry/difference-between-electronegativity-and-electron-affinity
- https://byjus.com/chemistry/difference-between-electronegativity-and-electron-affinity/
- https://collegedunia.com/exams/difference-between-electronegativity-and-electron-affinity-chemistry-articleid-5085
- https://pediaa.com/difference-between-electronegativity-and-electron-affinity/
- https://testbook.com/chemistry/difference-between-electronegativity-and-electron-affinity
- https://circuitglobe.com/difference-between-electronegativity-and-electron-affinity.html


excellent explanation!!