Diethyl ether, often known as ether, is an organic molecule composed of two ethyl groups connected by an oxygen atom as C2H5 – O – C2H5. It is a well-known anesthetic and is also known as ethyl ether. The IUPAC name for diethyl ether is ethoxyethane.
Valerius Cordus, a German physicist, botanist, and pharmacologist, developed this chemical in 1540. It was also known as the “sweet oil of vitriol” (oleum dulce vitrioli) because it is made by distilling a mixture of ethanol and sulfuric acid. August Sigmund Frobenius gave the chemical the name Ether in 1729. It is a highly flammable, colorless, volatile liquid with an ethereal odor. It’s a popular solvent for bromine, iodine, most fatty and resinous compounds, volatile oils, and pure water.
Interesting Science Videos
Structure of diethyl ether
An ether group is made up of an oxygen atom linked to two ethyl groups. They are represented by the formula C2H5 -O- C2H5. Bond angles of 104.5 degrees characterize the C-O-C connection, with C-O distances of around 140 pm. The ether’s oxygen is more electronegative than the carbons. As a result, the alpha hydrogens in the chain are more acidic than in typical hydrocarbon chains.
Preparation
By heating a mixture of ethyl alcohol and alumina
Ethyl alcohol on heating with alumina (Al2O3) at 250°C produces diethyl ether.
C2H5OH + HOC2H5 Al2O3 → C2H5 – O – C2H5 + H2O (at250°C/ Al2O3)
From Williamson method
In this process, Sodium or potassium ethoxide is heated with chloro ethane, bromo ethane, or iodo ethane to produce diethyl ether.
C2H5ONa + C2H5Cl → C2H5OC2H5 + NaCl
From silver oxide
The reaction of ethyl chloride with dry silver oxide (Ag2O), produces ether.
Ag2O + 2 C2H5Cl → 2 AgCl + C2H5OC2H5
Laboratory preparation
In the laboratory and on a big scale, it is produced by heating an excess of ethyl alcohol with concentrated sulphuric acid to 140 oC. The reactions involved are:
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5HSO4 + C2H5OH → C2H5 – O – C2H5 + H2SO4
A small amount of concentrated sulfuric acid can convert a large amount of alcohol into ether. As a result, this approach is also known as the Continuous etherification method.
Procedure
In a 250 ml distillation flask, 50 ml of conc. H2SO4 is gently mixed with 100 ml of pure alcohol with continuous shaking. The flask is then equipped with a drop funnel, a thermometer, and a water condenser that is connected to a receiver immersed in ice-cold water. The entire system is made airtight.
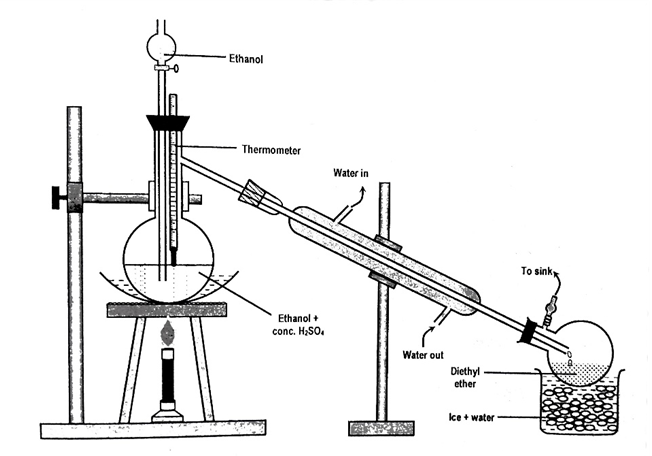
Fig: Laboratory preparation of diethyl ether
Image source: https://www.askmattrab.com/notes/350-Lab-Preparation-of-Ethoxyethane
The flask is now heated in a sand bath. When the temperature of the contents of the flask exceeds 140 oC, ether begins to distill over.
Impurities in the ether produced through this process include alcohol, water, and sulfuric acid. To eliminate the sulfuric acid, the resulting ether is rinsed with a sodium dioxide solution.
Ether is combined with a 50% calcium chloride solution and left to produce crystals of CaCl2.3C2H5OH in order to remove the alcohol. They are filtered and separated.
Then anhydrous calcium chloride is mixed with the ether to eliminate the water. The ether is then distilled at 35 °C to produce pure ether.
Physical properties of diethyl ether
- Diethyl ether is an odorless, colorless, highly volatile liquid with a sweetish-burning taste.
- Its boiling point is 34.6 °C.
- The melting point is -116.3 °C.
- It has a density of 0.7134 g/cm3.
- It is less soluble in water but more soluble in organic solvents including alcohol, benzene, and chloroform.
- It burns with a light flame.
- At 20 °C, the vapor pressure of Diethyl Ether is 439.8 mm Hg.
- It has polar nature.
Reactions of diethyl ether
Combustion
As it is more flammable, it burns more rapidly and generates an explosive combination with air.
C2H5 – O – C2H5 + 6O2 → 4CO2 + 5H2O
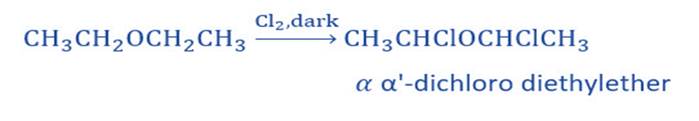
Halogenation
It produces a substituted product when it reacts with chlorine and bromine. Substitution on alpha-carbon atoms is facilitated, which means that hydrogen is substituted first from carbon next to an oxygen atom, and then other hydrogen atoms are substituted depending on the amount of the agent and other variables.
Peroxide formation
In the presence of sunlight, diethyl ether reacts with atmospheric oxygen to produce ether peroxide due to the presence of a lone pair of electrons on the ethereal oxygen.
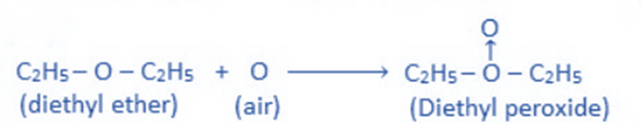
Reaction with conc. Sulphuric acid
The diethyl ether on heating with concentrated sulfuric acid produces ethyl hydrogen sulfate.
C2H5 – O – C2H5 + H2SO4 → C2H5HSO4 + C2H5OH
C2H5OH + H2SO4 → C2H5HSO4
Reaction with water
Ethyl alcohol is produced when diethyl ether is decomposed in water.
C2H5 – O – C2H5 + H2O → 2 C2H5OH
Reaction with HI
Ethyl iodide and ethanol are formed when diethyl ether reacts with cold and concentrated hydroiodic acid.
C2H5 – O – C2H5 + HI → C2H5I + C2H5OH
Reduction
In the presence of red phosphorous diethyl ether reacts with hydroiodic acid to give ethane.
C2H5 – O – C2H5 + 4H → 2C2H6 + H2O
Oxidation
It reacts with strong oxidizing agents like acidic potassium dichromate, to give acetaldehyde and acetic acid.
C2H5 – O – C2H5 + 2 (O) → 2 CH3CHO + H2O
CH3CHO + (O) → CH3COOH
Reaction with carbon monoxide
In the presence of boron trichloride, diethyl ether reacts with carbon mono oxide to give ethyl propanoate. This reaction is usually carried out at about 500 atmospheric pressure and about 100°C temperature.
C2H5 – O – C2H5 + CO → C2H5 – CO – O – C2H5
Reaction with acetyl chloride
It produces ethyl chloride and ethyl acetate when heated with acetyl chloride in the presence of anhydrous zinc chloride.
C2H5 – O C2H5 + CH3COCl → C2H5Cl + CH3COOC2H5
Uses of diethyl ether
- Diethyl ether is a common aprotic solvent used in laboratories.
- As diethyl ether has high volatility and low flash point, it is used as a starting fluid in gasoline and diesel engines.
- By combining ether with alcohol, Power alcohol can be used as a fuel in place of gasoline.
- It is employed as a solvent and medium in the production of Grignard reagents as well as in the Burtz reaction.
- It is used to cure hiccups by instilling it into the nasal cavity.
- It works well as a solvent for alkaloids, colors, fats, oils, resins, and waxes.
- It is used in the cellulose acetate and plastic industries to extract acetic acid from aqueous solutions.
- Diethyl ether usually superseded chloroform as a general anesthetic due to ether’s superior therapeutic index, or a larger gap between an effective dose and a potentially toxic dose.
Health hazards of diethyl ether
- Many diseases or health problems can result from inhaling diethyl ether, including nausea, vomiting, headache, cough, sore throat, labored breathing, and loss of consciousness.
- When it comes into contact with the eyes or skin, it produces irritation, which leads to skin dryness or cracking.
- High levels of Diethyl Ether exposure may also harm the kidneys.
- It is a highly flammable liquid and vapor that can also produce explosive peroxides.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://www.vedantu.com/chemistry/diethyl-ether
- https://byjus.com/chemistry/diethyl-ether/.
- https://www.britannica.com/science/ethyl-ether
