Diastereomers are stereoisomers, which are molecules that cannot be superimposed and are not mirror images of each other. As a result, they are not mirror images or identical stereoisomers. Diastereomers exist when two or more stereoisomers of the same substance have distinct configurations at one or more corresponding stereocenters and are not mirror reflections of one another.
So, diastereomers (also called diastereoisomers) are a form of stereoisomer. Diastereomers are non-identical, non-mirror image stereoisomers. As a result, they occur when two or more stereoisomers of a compound are not mirror images of one other and have distinct configurations at one or more (but not all) of the equivalent (related) stereocenters. Epimers are diastereoisomers that differ from each other at only one stereocenter.
Non-superimposable image: No matter how many rotation operations you perform on non-superimposable molecules, they will never appear exactly the same. The “rotations” include free rotation around single bonds as well.
Stereocenter: A stereocenter is an atom in a molecule that is connected to four separate atoms or groups of atoms. A stereocenter is often a carbon atom. Each stereocenter generates two distinct configurations, increasing the number of stereoisomers by a factor of two.
Enantiomers of a compound having more than one stereocenter are diastereomers of the compound’s other stereoisomers that are not their mirror image (except the opposing enantiomer). Diastereomers, unlike enantiomers, have varied physical qualities and, in many cases, different chemical reactivity.
Interesting Science Videos
Condition of diastereoisomers
I. They must have at least two stereocenters.
II. There must be non-superimposable.
III. They must be non-mirror images of one other.
Properties of diastereomers
i. Diastereomers have different physical characteristics such as refractive index, melting point, and boiling point.
ii. They have different chemical properties. For example, maleic acid is easily dehydrated, and fumaric acid is resistant to dehydration.
iii. They may be separated simply using chromatography or crystallization.
iv. Both optical activity and inactivity are possible.
v. They react at different rates and have varied solubilities.
vi. Diastereomers have a number of stereocenters. (at least two)
vii. Their bond lengths, bond angles, and torsional angles differ.
viii. They, like enantiomers, rotate plane-polarized light in opposite directions.
Types of diastereomers
Cis and trans diastereomers
Cis-Trans diastereomers or geometric isomers are formed when a molecule undergoes constrained rotation, most typically at a carbon-carbon double bond. Due to the limited rotation at the double bond, groups connected to it could be on the same or opposite sides of the alkene, resulting in stereoisomers.
If both of the double-bonded atoms have distinct substituents, molecules with carbon-carbon and carbon-nitrogen double bonds will exist as two different geometric diastereomers. These diastereomers are distinguished by the terms cis and trans, as well as Z and E (from the German zusammen for together and entgegen for opposing).
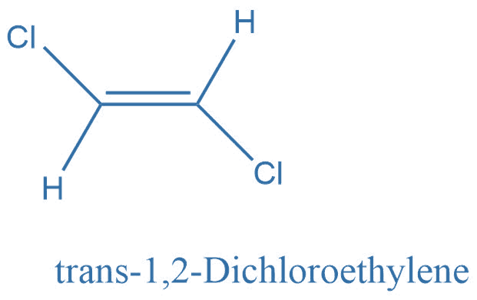
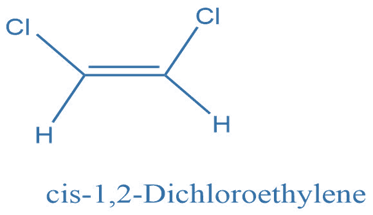
The Z isomer specifies the orientation when the two substituents with the highest priority are on the same side of the double bond. The Cahn, Ingold, and Prelog rules are used to establish the priority of each double-bonded atom’s substituents in potentially problematic situations.
The E-Z Notational System
E: higher priority ordered substituents on opposite sides
Z: higher priority ordered substituents on the same side
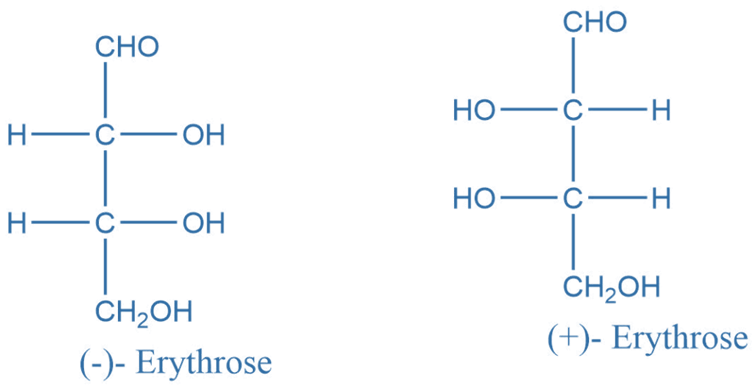
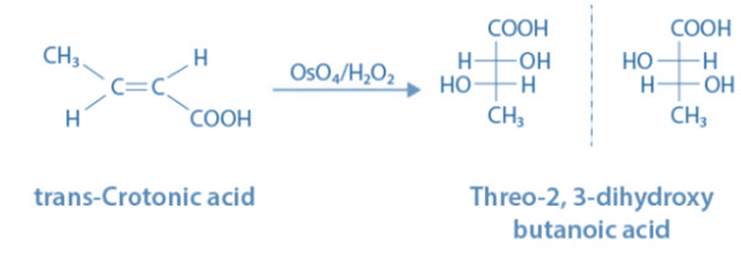
Erythro and thero diastereomers
They are geometrical isomers or compounds containing two or more chiral centers. Erythro arises when a diastereomer’s Fischer projection reveals comparable groups on the same side of the molecule. When similar groupings are on opposing sides of the Fischer projection, it is referred to as threo.
Trans-crotonic acid hydroxylation yields two enantiomers of threo-2,3-dihydroxy butanoic acid, whereas cis-crotonic acid hydroxylation yields erythro enantiomers.
Cahn, Ingold, and Prelog rules (CIP rules)
Stereoisomers share the same connection but differ in the spatial arrangement of their atoms. As a result, employing solely the IUPAC naming system results in the identical name for a pair of stereoisomers. It’s a concern for two reasons:
i. Each molecule should have a unique name, and
ii. a chemist who understands the naming system should be able to generate a structure for the molecule solely based on the name.
In addition to the IUPAC system, the Cahn-Ingold-Prelog system (or R, S-system), named after organic chemists Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog, overcomes these challenges. The Cahn-Ingold-Prelog system (or CIP) is a naming method used in combination with the IUPAC system to identify enantiomeric structures just by name.
Cahn, Ingold, and Prelog rules (CIP rules)
Rule 1: If four atoms linked to the stereocenter are different, and their priorities are determined by their atomic numbers.
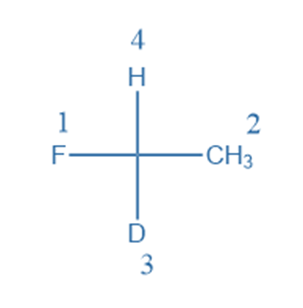
Rule 2: If two atoms are isotopes of the same element, the atom with the greater mass number takes priority over the other.
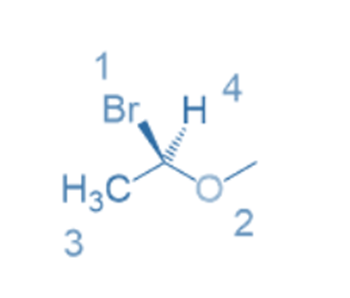
Rule 3: If the relative positions of two groups cannot be identified as previously, they are determined by a priority atom comparison at the first point difference.
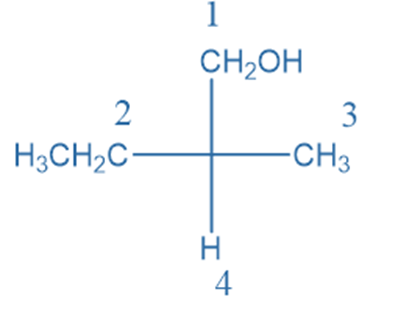
Rule 4: If two carbon chains are linked to the stereogenic carbon, the secondary carbon chain takes priority over the first carbon chain.
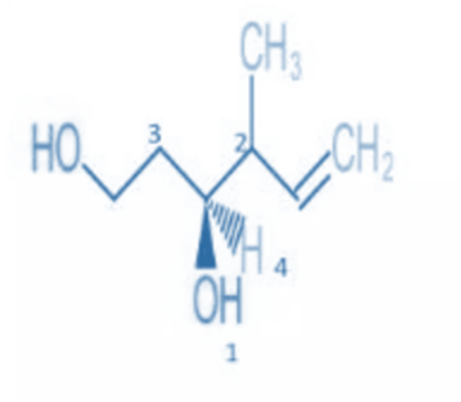
Rule 5: In the case of a double or triple bond, both atoms are regarded as duplicated or triplicated.
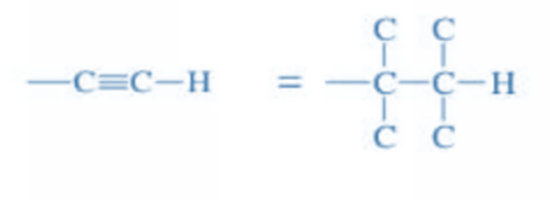
Rule 6: The molecule must be observed with the lowest priority group at the back to assign R and S configuration.
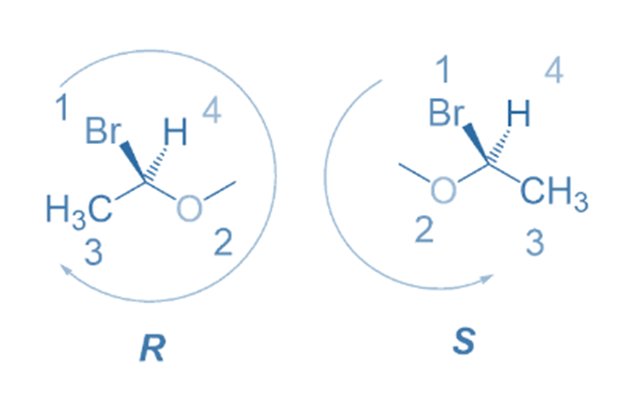
Rule 7: When the lowest priority group is facing the eyes, adjusting the location of any two groups twice at the stereocenter retains the configuration and also moves the lowest priority group away from the viewer.
Rule 8: In the case of compounds of the type abC=Cab, the isomer with identical groups on the same side of the double bond is named cis, while the isomer with identical groups on the other side is called trans.

Rule 9: If the two highest priority groups are on the same side of a double bond, the configuration is Z; if they are on opposite sides, the configuration is E.
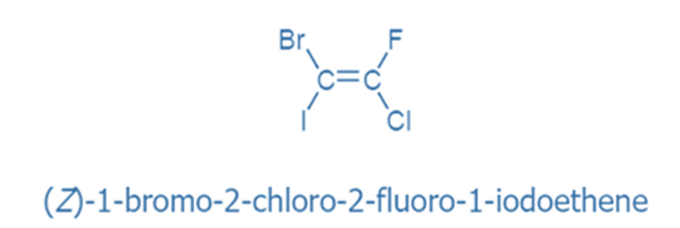
Assigning R and S configuration
S = Sinister” (Latin= “left)
R = “Rectus” (Latin= “right)
Cahn, Ingold, and Prelog’s rules determine the absolute configuration, R- and S-, of each chiral center in a chemical. The chiral centers are denoted by the letters R- or S-.
Identification of the R and S configuration of the chiral center by using the Cahn-Ingold-Prelog rules includes different steps. They are:
1) Find a carbon that has four separate groups linked to it.
2) Position the lightest group in the back (pointing away from you).
3) Assign the highest priority (number 1) to the group with the most connections ( or high atomic number).
4) Give the number 2 to the second heaviest group.
5) Assign the number 3 to the third heaviest group.
6) If, with the lightest group or atom pointing away from you, the highest priority to the lowest priority (1→2 → 3) goes clockwise, the center is known as R-, and if it goes counterclockwise, it is known as S.

Diastereomeric excess
Diastereomeric excess is similar to enantiomeric excess. It is an excess of one of the diastereomers over the other. It is employed for quantitative purposes. When a sample contains a combination of diastereomers, their concentrations may vary.
It quantifies the extent to which a sample contains more of one diastereomer than the other. Diastereomeric excess aids in assessing stereoselectivity by indicating that when a chiral auxiliary is eliminated, the product’s enantiomeric excess equals the diastereomeric excess. The diastereomeric excess calculation also helps in the computation of purity measurement for chiral compounds. The diastereomeric excess is calculated using the following formulas:
de = [a] – [b] / [a] + [b] where [a] and [b] are two diastereomeric
Resolution of diastereomers
Diastereomers are stereoisomers that are neither identical nor superimposable to one another. They differ in their physical and chemical features. As a result, they can be easily separated by employing various separation strategies such as
i. Recrystallization
ii. Chromatography
iii. Distillation
Applications of diastereomers
Two diastereomers will not have the same chemical characteristics. In chiral synthesis, this knowledge is used to separate a mixture of enantiomers. This is the underlying principle of chiral resolution. After the diastereomers have been prepared, they are separated using chromatography or recrystallization. It also considers the stereochemistry of enol and enolate ketonization.
Difference between Enantiomers and Diastereomers
| S.N | Property | Enantiomers | Diastereomers |
| 1. | Definition | Enantiomers are stereoisomers that are not superimposable mirror images of each other. | Diastereomers are stereoisomers that are not superimposable, non-mirror images of each other. |
| 2. | Occurrence | They are present in pairs. | There can be several molecules in diastereomers. |
| 3. | Characterization | Molecules with more than one stereocenter can be diastereomers if they are not mirrored images of each other. | If there is only one stereo center, the molecule possesses enantiomers. |
| 4. | Solubility | They have identical solubilities. | They have different solubilities. |
| 5. | Chemical and physical properties | They have similar chemical and physical properties. | They have different chemical and physical properties |
| 6. | Optical rotation | Enantiomers rotate the plane of polarised light in opposite directions to an equal extent. | They can be optically active or inactive. The optically active diastereomers do not rotate plane-polarized light in opposite directions to an equal extent. |
| 7. | Chirality | They are chiral compounds. | They may be chiral or achiral. |
| 8. | Molecular shape | They have the same molecular shape. | They have different molecular shapes. |
| 9. | Reaction rate | Enantiomers have the same reaction rates. But they react with optically active reagents at different rates. | They have different reaction rate |
| 10. | Bond angle and bond length | They have the same bond angle and bond length | They have different bond angles and bond lengths. |
| 11. | Separation | Due to similar physical and chemical properties, they are difficult to separate. | They can b easily be separated by crystallization, fractional distillation, chromatography technique, etc. |
References
- K.R palak,2017, Stereochemistry. Pairavi Prakashan.
- http://what-when-how.com/molecular-biology/diastereomer-molecular-biology/.
- https://www.organicchemistrytutor.com/lessons/enantiomers-and-diastereomers/.
- Organic-synthesis-strategy-and-control-by-stuart-warren11..
- https://chem.libretexts.org/Courses/Purdue/Purdue%3A_Chem_26505%3A_Organic_Chemistry_I_(Lipton)/Chapter_3._Stereochemistry/3.6_Cahn-Ingold_Prelog_Rules.
