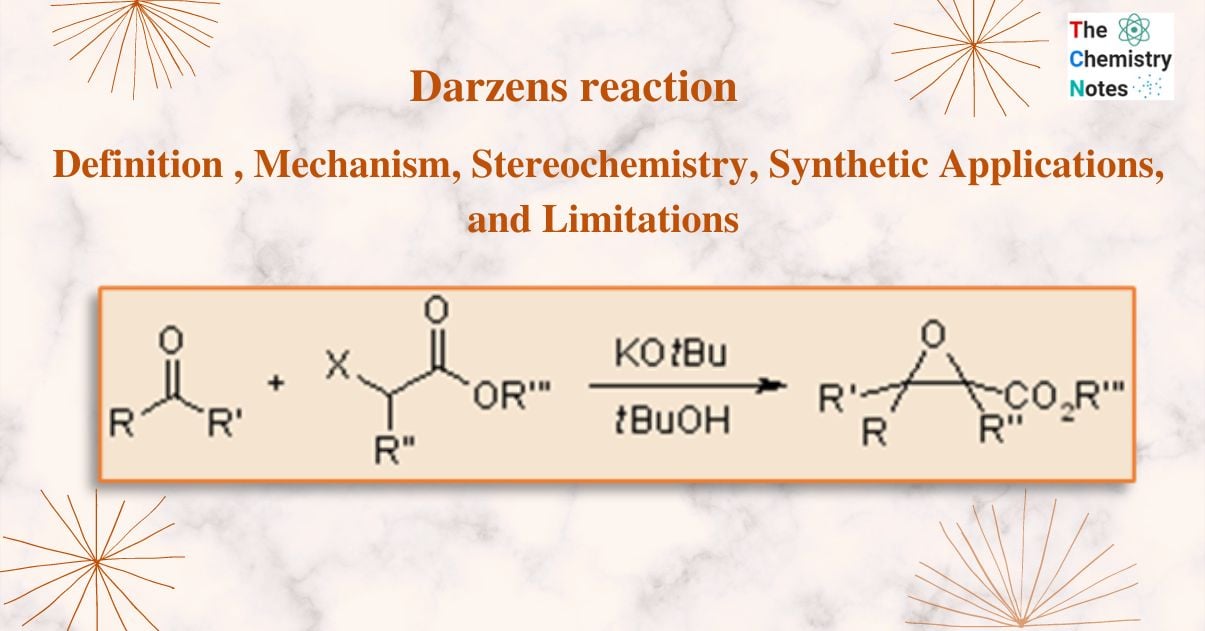
Darzens reaction is a condensation reaction that involves the reaction of ketone or aldehyde with an α-haloester in the presence of a base to form an α,β-epoxy ester i.e., glycidic ester.
It is commonly known as Darzens condensation or glycidic ester condensation. This reaction is named after the organic chemist Auguste Georges Darzens in 1904.
Sodium ethoxide is typically employed as an effective condensing agent. Aliphatic aldehyde yields are low, most likely due to the formation of aldol compounds, whereas aromatic aldehydes and ketones or, α, β -unsaturated ketones provide good glycidic ester yields.
Interesting Science Videos
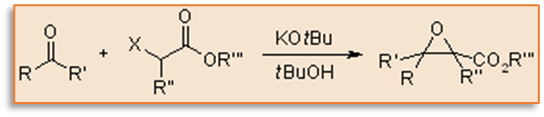
Reaction mechanism of Darzens reaction
The formation of a carbanion occurs at the halogenated position when a strong base is utilized. This carbanion forms quite readily because the ester makes it a resonance-stabilized enolate. This enolate attacks another carbonyl component, forming a new carbon – carbon bond. The formerly nucleophilic halide-bearing site is then attacked by the oxygen anion via an intramolecular SN2 reaction, removing the halide to produce an epoxide. The ester’s main function is to make the initial deprotonation possible. Alternatives include using the other carbonyl functional groups. The end product is an,-epoxy amide if the starting material is a -halo amide.
Stereochemistry of Darzens reaction
The step when the carbanion attacks the carbonyl establishes the initial stereochemistry of the reaction chain. At this point, two SP3 (tetrahedral) carbons are formed, allowing for the formation of two distinct diastereomeric forms of the intermediate halohydrin.
The initial stereochemistry of the reaction sequence is established in the step where the carbanion attacks the carbonyl. Two SP3 (tetrahedral) carbons are created at this stage, which allows two different diastereomeric possibilities of the halohydrin intermediate. Due to chemical kinetics, the main product of this reaction will most likely be the one that is easiest and fastest to generate. The cis or trans form of the epoxide is regulated by the kinetics of an intermediate step since the succeeding SN2 reaction step occurs with stereochemical inversion. Due to the basic nature of the reaction conditions before the SN2 reaction, the halohydrin can alternatively epimerize. In this situation, the first created diastereomer may change into another. The cis or trans form of the epoxide is regulated by chemical thermodynamics in this equilibrium process i.e., the product of the more stable diastereomer, regardless of which one was the kinetic consequence.
Applications
- The ester may be hydrolyzed leading to decarboxylation, which causes the epoxide to rearrange into a carbonyl.
- This reaction also can be used for the Synthesis of α-pinene.
- Epoxides with electron-withdrawing groups are produced through the non-oxidative Darzens reaction.
Limitations
- Acetaldehyde and monosubstituted acetaldehyde derivatives cannot be produced using this process, most likely because the aldehyde self-condenses under the influence of a competing base.
- High yield in the primary reaction product is a desirable characteristic that a reaction must satisfy to be eligible for a kinetic investigation. Unfortunately, in the Darzen condensation, yields of this nature are not typical.
Read also:
- Cope rearrangement reaction
- Favorskii rearrangement
- Finkelstein reaction
- Balz–Schiemann Reaction
- Etard Reaction
- Hydroboration-Oxidation Reaction
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
