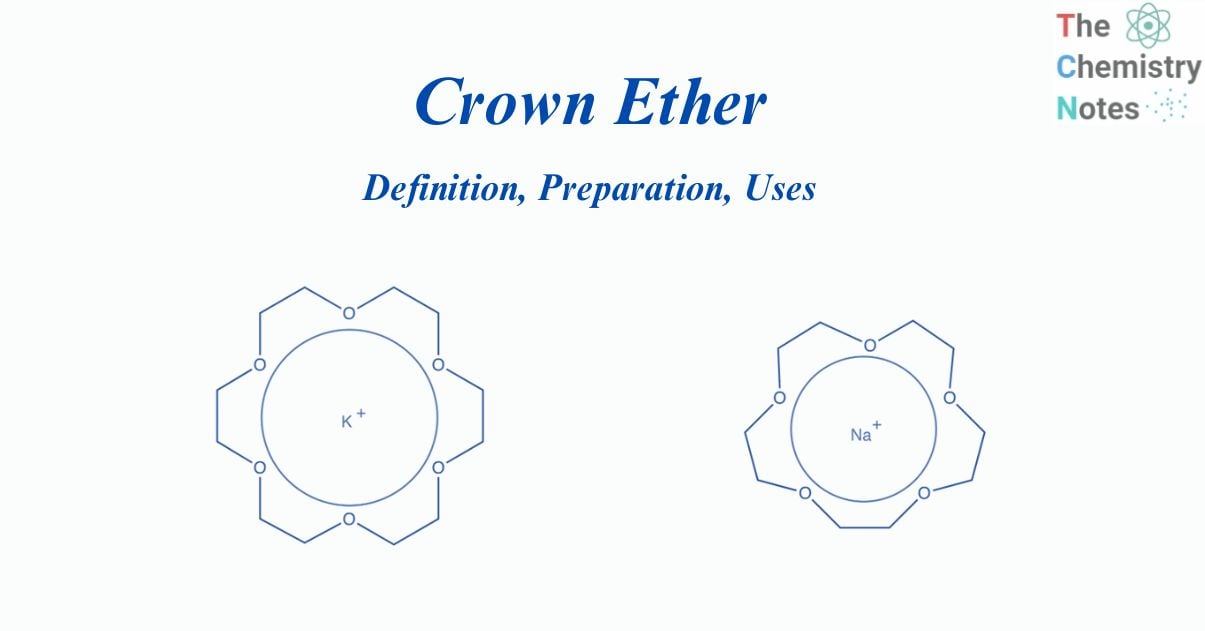
Crown ethers are heterocyclic chemical compounds composed of a ring with several ether groups. The most frequent crown ethers are ethylene oxide oligomers with the repeating unit ethylene oxy (-CH2CH2O-). They have the general formula (OCH2CH2)n or (OCH2CH2CH2)n.
Pederson synthesized a sequence of macrocyclic polyethers, which are cyclic compounds with four or more oxygens in a ring with 12 or more atoms. Because their molecular structures resemble crowns, he named these substances crown ethers.
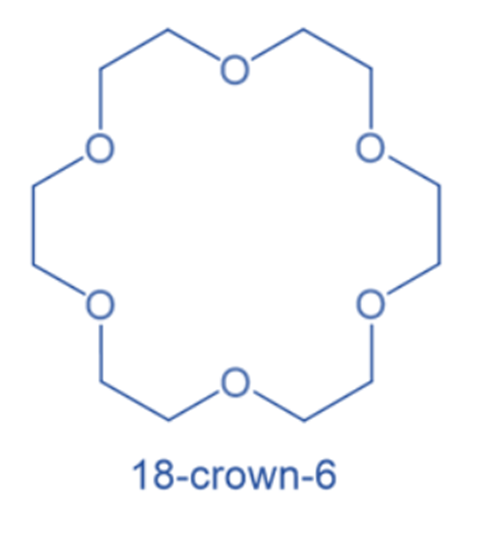
Crown ether (CE) nomenclature is quite complicated, thus Pederson proposed a shorthand definition in which the word crown is preceded by the total number of atoms in the ring and is followed by the number of oxygen atoms. As a result, 18-crown-6 is an 18-membered ring containing six oxygen atoms. CE are cyclic chemical compounds composed of a ring with many ether groups.
The most prevalent CEs are ethylene oxide oligomers. The tetramer (n = 4), pentamer (n 5), and hexamer (n 6) are all important members of this series. The term “crown” refers to the structural similarity between a crown ether bonded to a cation and a crown sitting on a person’s head.
A hollow at the center of all crown ethers is lined with oxygen atoms and can form complexes with positive ions, most notably metallic ions or ammonium and substituted ammonium ions. The cation is stabilized by interacting with lone pairs of electrons on the oxygen atoms around it. Depending on the size of its cavity, a crown ether bonds certain metal ions or organic molecules.
Interesting Science Videos
Nomenclature of crown ether
Crown ethers are classified according to IUPAC nomenclature as well as by short names (Pedersen’s crown nomenclature). Their name is usually given by the notation. For example: (18)-crown-6. The first number denotes the ring size, while the second denotes the number of oxygen atoms in the ring.
In addition to oxygen, other heteroatoms such as sulfur and nitrogen may be present.
If S is present as a heteroatom, they are known as thia crown ethers; if N is present as a heteroatom, these are known as aza crown ethers.
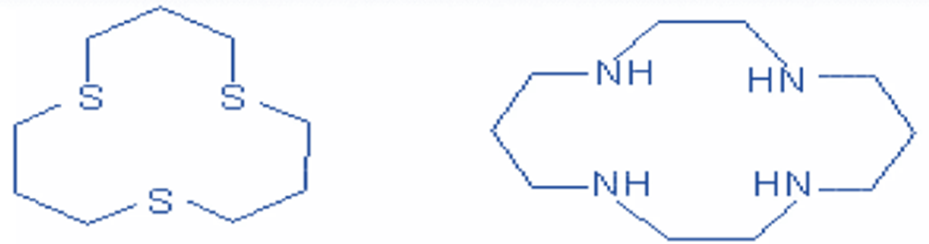
Thia and aza crown ethers
Types of crown ether
Crown ethers are classified into two types: aliphatic and aromatic. The most prevalent aliphatic CEs are cyclic oligomers of ethylene oxide that contain four to ten -(CH2CH2O)- repeating units linked together; one or more ethylene groups of the macrocycle may be part of an aliphatic moiety such as cyclohexyl. The presence of endocyclic aromatic rings in the structure of aromatic CEs distinguishes them. Although many aromatic moieties have been added to aromatic CEs, the benzene ring is by far the most prevalent, giving rise to benzo crown (BCE) and dibenzo crown (DBCE) ethers.
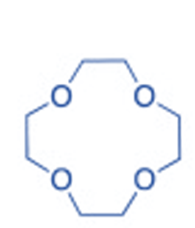
Aliphatic crown ether ( 12crown-4)
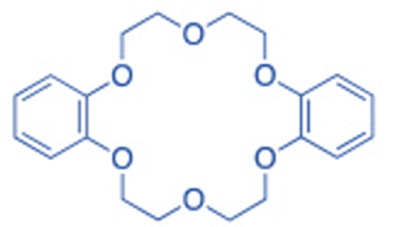
Aromatic crown ether (Dibenzo 18 crown-6)
Preparation of Crown ether
Cyclization of acylic compounds
The synthesis of crown ether incorporates four processes described by Pedersen employing high dilution procedures that undergo cyclization of acyclic compounds.
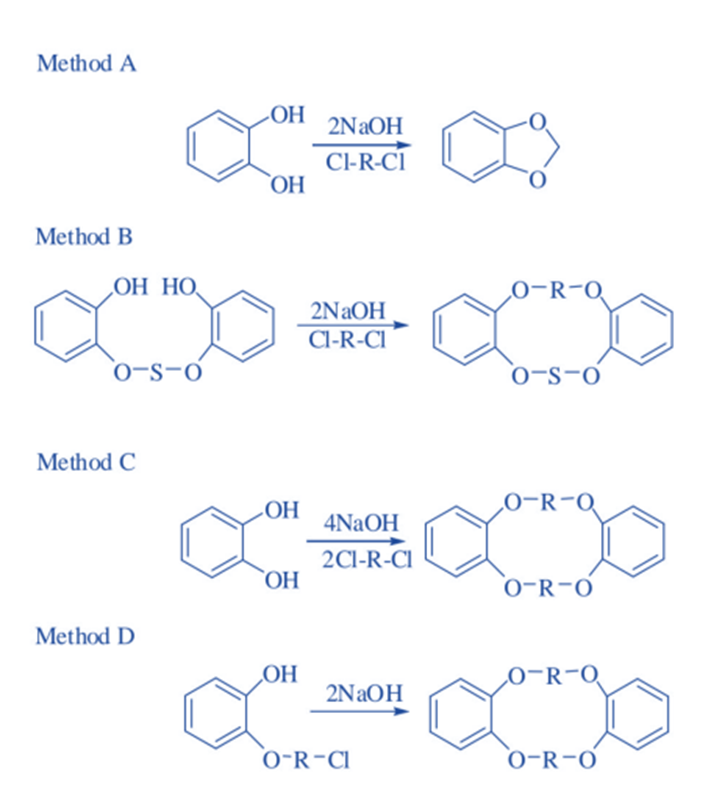
Template method
The main drawback of direct techniques is the absence of effective, carefully explained, and independently tested protocols and processes.
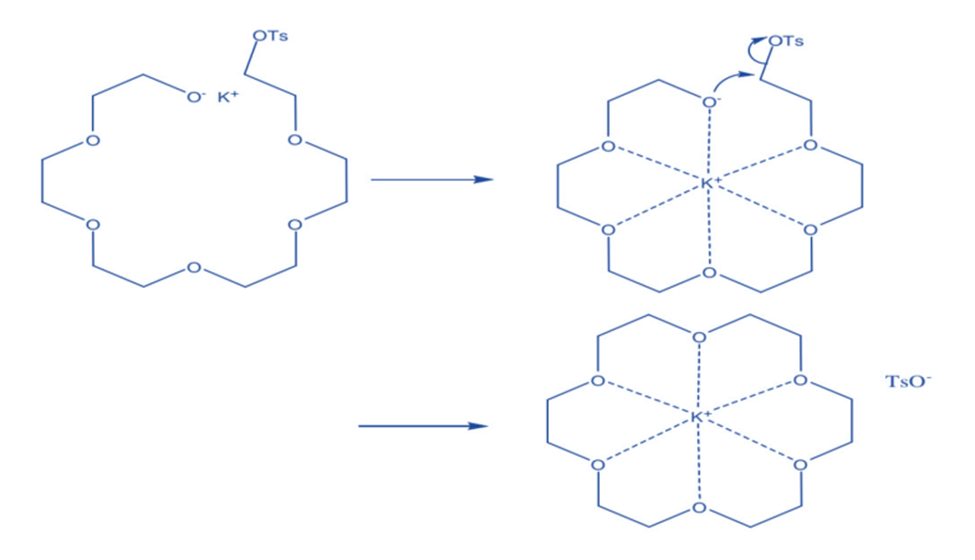
Templated techniques rely on the size complementarity of the target macrocycle’s cavity and an additional metal cation. The latter serves as a template by arranging the polyether chain precursor into the precise conformation necessary for successful cyclization, hence reducing the generation of polymeric side products. As a result, Li+ templates the macrocyclization of 12-membered CE, whereas Na+, K+, and Cs+ prefer 15-, 18-, and 24-membered cycles, respectively.
Aside from standard approaches, Dale et al’s ‘template effect’ in double Williamson synthesis of 18-Crown-6 provides a stimulating process for the synthesis of these complexes. The presence of a sufficient size cation as a template aids in the synthesis of crown ether rings by holding the partially formed ligand in place. This is known as the template effect. The template cation and the acyclic molecule have a binding relationship. The association between ligand and cation template is modest, whereas cation interactions with ethereal oxygen are strong.
Mandolinell investigated the template effect further for the cyclization of starting material to synthesize 18- Crown-6 ether utilizing various hydroxide bases.
Synthesis of Bis-benzo-15-crown-5 Ether
This synthesis began when a solution of molecule 1,2-bis(bromomethyl)benzene and ethanol reacted with a solution of hydroxyl benzaldehydes and sodium hydroxide. The major reactant formyl-substituted compounds for the final production of bis-crown ethers were synthesized in this procedure.
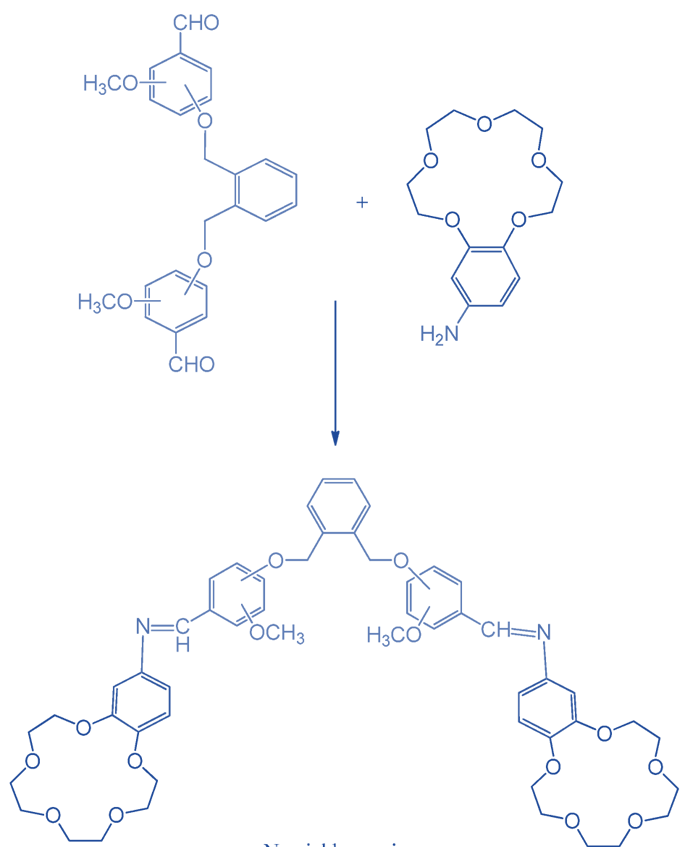
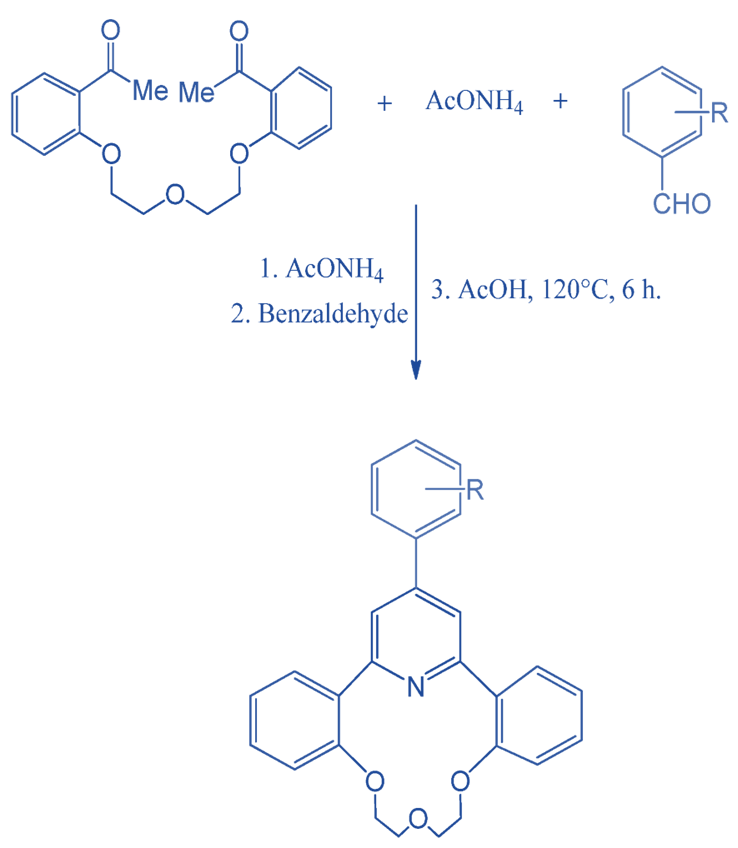
Synthesis of (γ-Arylpyridino)-dibenzo aza-14-crown-4 Ether
To synthesize (γ-arylpyridino)-dibenzoaza-14-crown-4 ether, a domino-type condensation approach was used. The reaction was carried out at 120 °C for 6 hours. The three principal components employed were an aromatic aldehyde, ammonium acetate, and 1,5-bis-(2-acetyl phenoxy)-3-oxapentane.
Chemistry of crown ether
In the molecular structure of crown ethers, the hydrophobic ethylenic group surrounds the oxygen atoms in the hydrophilic (electron-rich) cavity. As a result of the presence of the hydrophobic ethylenic group, metals can be dissolved in organic solvents. The primary goal of designing diverse crown ethers is to differentiate between different chemical species. CEs’ complexing capabilities can be altered by altering the cavity size, substituents, and coordinating atoms in the cavity.
Crown ether’s complexing capacity is mostly determined by:
1. Cavity size
2. The cation’s size
3. the charge density
4. Counter ion nucleophilicity
5. The nature of the solvent
Crown ether: Host guest chemistry
This organic chemical class has an unusual structure, with a hydrophobic ring encircling a hydrophilic cavity. CEs are heterocyclic chemicals that exist in the form of cyclic oligomers. The unusual properties of CEs result from the graceful interplay of chemically stable and conformationally flexible ethylene units with nucleophilic oxygen atoms positioned symmetrically around the ring. This combination produces an electron-rich cavity capable of accommodating acceptable guests in addition to providing the compounds with a moderately amphiphilic character.
These have a hydrophobic ring encircling a hydrophilic cavity and can form stable complexes with metal ions, leading to host-guest chemistry. Since their development, they have demonstrated an exceptional capacity to selectively coordinate ions, making them appealing for a wide range of research applications. Complex compounds in which a metal cation acts as the guest and a CE act as the host are classified as host-guest chemistry. So, These are exceptionally versatile compounds with a higher affinity for metal ions, such as s-block and transition metal ions. For example, 18-crown-6 possesses a cavity the size of 4f transition metal ions and exhibits extraordinary affinity for complexation with lanthanide ions.
CEs’ ability to bind organic molecules or ions is determined by the size of their cavity. Since the diameter of a crown ether molecule’s cavity is more or less constant, a crown ether molecule’s ability to create a stable host-guest complex with a metal ion is highly selective.
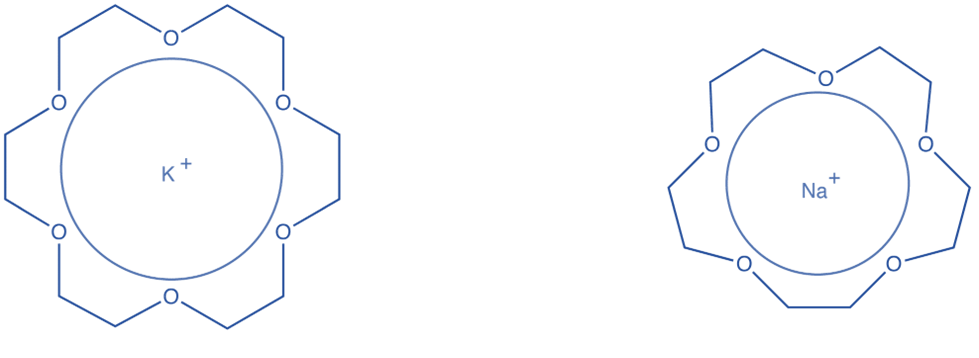
18-crown-6 cavity diameter > 15-crown-5
K+ has a greater ionic radius than Na+.
So, while 18-crown-6 forms a stable host-guest complex with K+ but not with Na+, 15-crown-5 forms a stable host-guest complex with Na+ but not with K+.
Crown ether: Phase transfer catalyst
Indeed, CEs can selectively bind alkali and alkaline earth ions, generating complexes that are soluble in non-polar solvents due to their lipophilic nature, acting as carriers of the charged species. As a result, they’ve seen a lot of applications in phase transfer catalysis and ion extraction. They have lately been used as ion translocators and as components of synthetic channels via lipidic membranes. It has also been shown that CE’s complexing capacity is not restricted to metal cations, as they may form stable supramolecular complexes with a wide range of organic guests in an aprotic solvent. In addition to this, crown ethers were employed for a variety of purposes, including solvent extraction, in which the organophilic salt used was picrate.
Applications of crown ether
- CE-based sensors were used to measure serotonin in human serum.
- Dibenzo 24-crown-8-ether-based potentiometric sensors are used to determine Biperiden hydrochloride in urine and plasma. Biperiden is used to treat Parkinson’s disease.
- CEs are crucial in conducting catalytic activity in many phase transfer processes. The usage of monosaccharide-based crown ethers in chalcones epoxidation has led to a better knowledge of the enantioselectivity of compounds that are important in drug discovery.
- Several simple crowns demonstrated the ability to interact with enzymes, including R-chymotrypsin, lipases, and subtilisin Carlsberg, which profited from the presence of these CEs. CEs gained popularity due to their distinct structure and uses in chemical synthesis and medicinal delivery. CEs’ ionophoric capabilities allow them to pass past membranes and interact with biological systems.
- Crown ethers are utilized as sensors in potentiometric ion selective electrodes. More than 200 such sensors have been recorded to date.
- Chiral crown ethers have been widely employed for the separation of diverse chiral compounds. Stationary phases derived from tetracarboxylic acid (18-C-6-TA) were used for the resolution of several types of analytes.
- Crown ethers have high selectivity and affinity for particular metals. As a result, they are effectively extracting agents for eliminating alkali metal salts from mixtures.
- Crown ethers are useful for separating cation combinations.
- (Because of their remarkable selectivity, crown compounds can find and wrap around a guest atom in a solution.) Crown ethers are commonly utilized in organic synthesis.
- Crown ethers can help with phase transfer catalysis.
- Crown ethers are commonly used in complex cations, amines, phenols, and other organic compounds.
References
- https://www.jetir.org/papers/JETIR1907N71.pdf
- https://annamalaiuniversity.ac.in/studport/download/sci/chemistry/resources/Crown%20ether%20notes-%20I%20MSc%20Chemistry%20(CBCS).pdf
- https://slideplayer.com/slide/12958980/.
- https://digitalcommons.colby.edu/cgi/viewcontent.cgi?article=1231&context=seniorscholars.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8477657/.
- http://www.ochempal.org/index.php/alphabetical/c-d/crown-ether/
