In the field of petroleum refining, cracking is a chemical process that involves the fragmentation of heavy hydrocarbon molecules into lighter molecules through the application of heat, pressure, and occasionally catalysts. The process of cracking holds significant importance in the realm of commercial gasoline and diesel fuel production. Cracking crude oil is used to convert long alkanes into shorter, more useful hydrocarbons.
Petroleum and its derivatives play a crucial role in our everyday existence. The process of extracting crude oil and natural gas from the earth involves transportation to refineries where it undergoes various physical and chemical alterations. The term “feed stocks” is commonly used in the petroleum industry to refer to the raw materials utilized in the production process. Within the refinery, the feed stocks undergo a process known as cracking, whereby intricate organic molecules are fragmented into lighter molecules that possess considerable commercial worth. Therefore, the act of cracking is a fundamental procedure in these particular domains.
Interesting Science Videos
What is Cracking?
Cracking is a process commonly employed in oil refineries to break down large and intricate hydrocarbon molecules into smaller and lighter components that have greater commercial or consumer value. The process of refining crude oil involves a crucial stage known as cracking.
The process of cracking holds significant importance in the functioning of oil refineries. The process of refining crude oil involves the utilization of a technique known as fractional distillation. This approach involves the separation of crude oil into multiple fractions, which are categorized according to the boiling points of their individual molecules. The fractions in question are heavy gas oil, lubricating oil, gas oil, diesel, kerosene, gasoline, naphtha, and gas, arranged in a specific sequence. The molecular length and configuration exhibit diverse properties, such as boiling point, due to their variability.
The process of cracking involves the decomposition of intricate organic molecules, such as long chain hydrocarbons or kerogens, into smaller molecules, specifically light hydrocarbons. The phenomenon is attributed to the cleavage of carbon-carbon bonds.
Cracking of Hydrocarbons
Cracking is a chemical process that involves the breakdown of larger saturated hydrocarbon molecules into smaller and more beneficial hydrocarbon molecules. This process also results in the formation of some unsaturated hydrocarbon molecules.
The initial hydrocarbons are alkanes. Cracking produces alkanes and alkenes, which are members of a distinct homologous series.
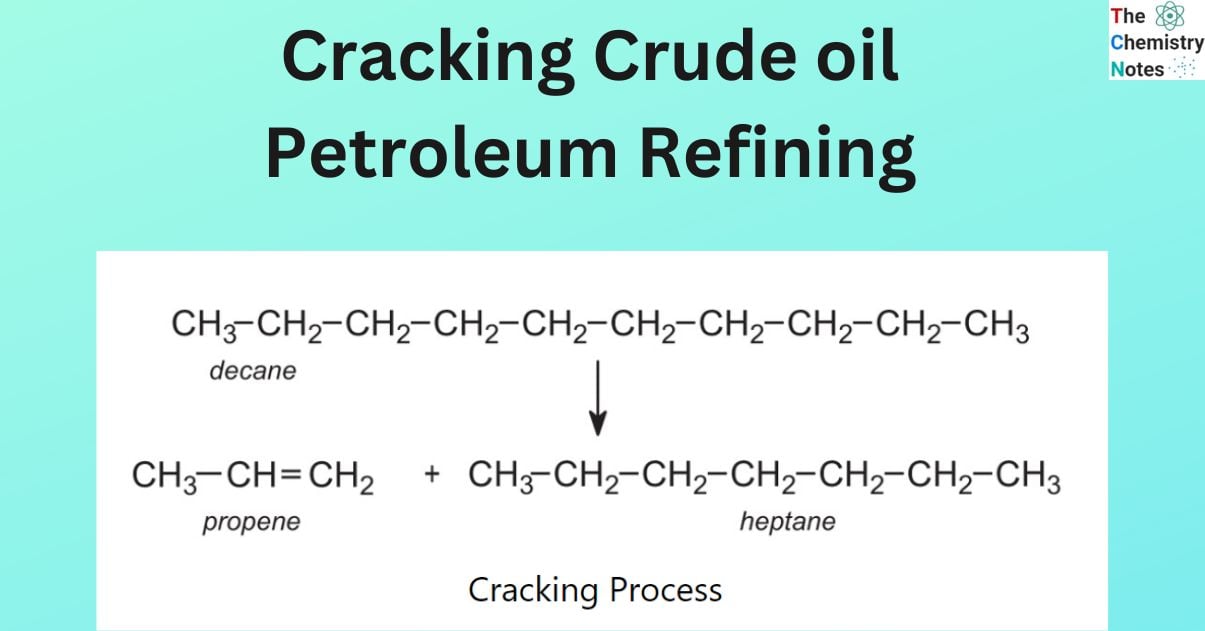
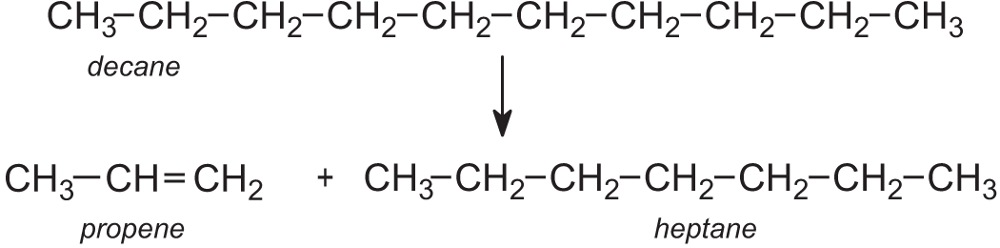
An example of a chemical reaction is the cracking of hexane to produce butane and ethene.
hexane → butane + ethene
C6H14 → C4H10 + C2H4
The initial compound will consistently adhere to the alkane rule of CnH2n+2. The initial product will also adhere to this principle. The second product will consist of all the remaining C and H atoms. Since the second product is an alkene, it will adhere to the CnH2n rule.
Types of Cracking in Petrochemistry
Thermal cracking
Thermal cracking is a process that involves breaking down complex long-chain hydrocarbons into lighter particles through the application of high temperature conditions. Free radicals are the active species responsible for governing thermal cracking reactions. These reactive species contain unpaired electrons but do not have an electronic charge. Gasoline with relatively low octane numbers can be produced through thermal cracking of gas oil due to the free radical chemistry involved.
- The first thermal cracking process for breaking up large nonvolatile hydrocarbons into gasoline came into use in 1913; it was invented by William Merriam Burton, a chemist who worked for the Standard Oil Company (Indiana), which later became the Amoco Corporation.
- Olefins are the primary products of thermal cracking.
- There are two known types of thermal cracking depending on the product range.
- One method for producing ethylene and other valuable feedstocks in the petrochemical industry is through steam cracking or pyrolysis. This process involves subjecting materials to high temperatures, typically between 750 and 900 degrees Celsius, in the absence of oxygen.
- The second type of temperature cracking is referred to as delayed coking, and it is conducted at a lower temperature range of approximately 500°C. Delayed coking produces a type of petroleum coke known as needle coke, which is highly crystalline. This material is utilized in the steel and aluminum industries for the production of electrodes. Modern high pressure thermal cracking typically operates at a pressure of 7,000 kPa.
Steam cracking
Steam cracking is a chemical process used in the petrochemical industry to break down large hydrocarbon molecules into smaller ones
- Steam cracker units are facilities designed to thermally crack feedstocks like naphtha, LPG, butane, ethane, and propane. This is achieved by using steam in a bank of pyrolysis furnaces.
- Steam cracking is a process that is carried out in the absence of oxygen.
- This method is the primary industrial process used to produce lighter alkenes such as ethene and propene.
- Steam cracking is a fundamental technology that underpins the largest chemical processes, namely the production of ethylene and propylene.
Catalytic Cracking
French chemist Eugène Houdry improved the cracking process with catalysts to obtain a higher-octane product. Catalytic cracking occurs at a lower temperature, resulting in energy savings. Due to its lower operating temperature, the production of alkene is minimized. Alkenes are known to induce instability in hydrocarbon fuels. It is divided into fluid catalytic cracking and hydrocracking.
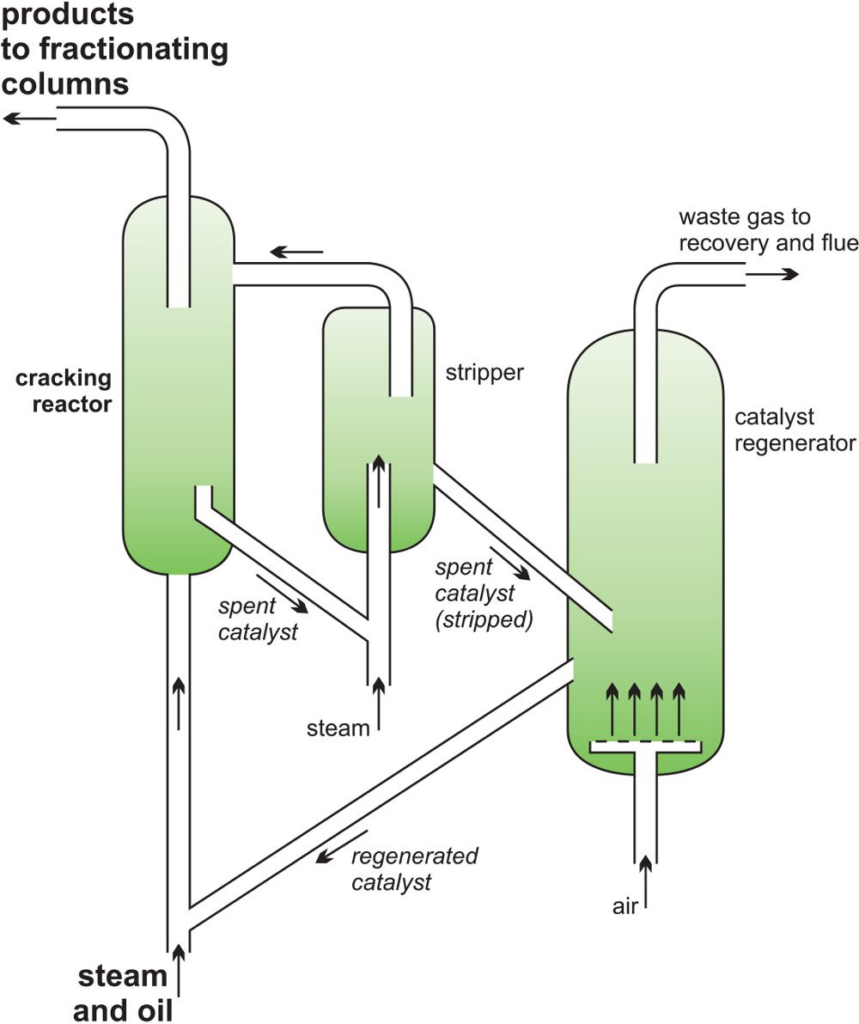
Fluid Catalytic Cracking (FCC)
FCC (Fluid Catalytic Cracking) is a process that converts high boiling point, high molecular weight crude oil into various petroleum products such as gasoline, olefinic acid, and others.
- Catalytic cracking was itself improved in the 1940s with the use of fluidized or moving beds of powdered catalyst
- Typically, the feedstock used is a dense gas oil with a molecular weight between 200 to 600 or higher, and a boiling point within the range of 340oC.
- During the FCC process, the heavy gas oil feedstock undergoes heating at high temperature and moderate pressure.
- It is then introduced into a bed of hot powdered catalyst, which effectively breaks down the long chain high boiling point hydrocarbon liquid into short chain molecules. The resulting vapors are collected for further use.
- Solid acid catalysts such as silica-alumina and zeolite are commonly used as catalysts in FCC.
Hydro Cracking
Hydrocracking is a process that involves the use of hydrogen and a catalyst to break down heavy molecules with high boiling points into gasolines and distillates.
- The catalysts commonly used for hydrocracking are Al2O3/SiO2 that have been impregnated with nickel, molybdenum, cobalt, or tungsten.
- The entire reaction takes place within a hydrocracker. Inside a hydrocracker, two primary reactions occur: the catalytic breakdown of heavy hydrocarbons into lighter unsaturated hydrocarbons, and the subsequent saturation of these newly formed hydrocarbons with hydrogen.
- Hydrocracking yields several significant petroleum products such as diesel, kerosene, jet fuels, and other related products. In addition, the process yields fractions of low sulfur naphtha and LPG.
Chemical Process of Cracking
The process of cracking chemicals involves a series of free radical reactions. The following are these cracking reactions:
Initiation: Free radicals play a crucial role in the process of cracking. A single molecule undergoes breakdown, resulting in the formation of two free radicals. The smaller fragment of the molecule then proceeds to undergo initiation.
CH3CH3 → 2CH3•
Hydrogen Abstraction: Hydrogen is removed from the second molecule, hence it becomes a free radical as shown.
CH3• + CH3CH3 → CH4 + CH3CH2•
Radical Decomposition: Free radicals break into other free radicals and alkane to produce alkene products.
CH3CH2• → CH2=CH2 + H
Radical Addition: Free radicals react with alkene and produce free radicals which form aromatic products.
CH3CH2• + CH2=CH2 → CH3CH2CH2CH2•
Termination: The termination process happens when two radicals react with each other to form a non-free radical product resulting in recombination and disproportionation.
CH3 + CH3CH2 → CH3CH2CH3
CH3CH2 + CH3CH2 → CH2=CH2 + CH3CH3
Cracking Equation with Examples
- The balanced equation for the cracking of C12H26 into pentane and another hydrocarbon.
Cracking equations follow this generic equation:

- Write the general equation for cracking
A long-chain hydrocarbon can be converted into a shorter alkane molecule and an alkene.
Long-chain hydrocarbon → shorter alkane molecule + alkene
- Complete the formulas. Since pentane is one of the molecules that is produced, we can accurately write its corresponding formula.
C12H26 → C5H12 + ?
- Calculate the number of carbon atoms. We can examine the number of carbons present on each side. There are 12 carbons on the left-hand side. There are five carbons on the right, indicating that the second hydrocarbon produced must contain seven carbons.
The chemical equation: C12H26 → C5H12 + C7H
- Calculate the number of hydrogen atoms. We now need to determine the number of hydrogen atoms. On the left-hand side, there are 26 hydrogens, whereas on the right-hand side, there are only 12. Thus, it can be concluded that the second hydrocarbon produced contains 14 hydrogen atoms. Thus, the second molecule can be identified as heptene, which is an alkene.
The chemical equation is: C12H26 → C5H12 + C7H14
- Predicting the Second Product of the Cracking of Heptane given the Displayed Formula of the Other Product
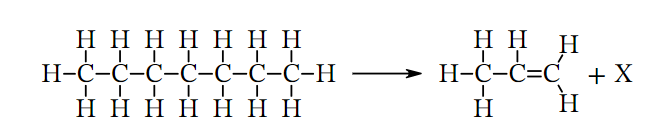
- Heptane is an alkane that has a chemical formula of C7H16. When an alkane undergoes a cracking reaction, the resulting products will be an alkane and an alkene. However, both of these products will be shorter in length compared to the original alkane.
- Propene, an alkene with the chemical formula C3H6, is the initial product formed in this cracking reaction.
- Based on this information, it can be inferred that the chemical formula for the second product is C4H10.The provided information indicates that the compound follows the typical formula for an alkane, which is CnH2n+2, where n equals 4 in this case.
- therefore it can be concluded that compound X is butane.
Importance of Cracking
- Smaller hydrocarbons such as gasoline are more efficient fuels compared to larger hydrocarbons. Cracking is a process that breaks down large hydrocarbons into smaller ones, making fuels more easily accessible. Maintaining a balance between supply and demand is facilitated by this.
- The supply refers to the percentage of oil that a refinery produces. Demand refers to the amount of a product or service that consumers are willing and able to purchase at a given price. During the process of fractional distillation of crude oil, a higher quantity of larger hydrocarbons that are marketable is produced, while a lower quantity of smaller hydrocarbons that are in demand by consumers is obtained.
- Maintaining a balance between fuel supply and demand is advantageous in today’s world. Breaking down the long-chain hydrocarbons obtained during fractional distillation in refineries is essential to produce efficient gasoline, as short-chain hydrocarbons have a high fuel efficiency.
- Cracking is a process that removes large hydrocarbon molecules from raw crude oil to produce by-products such as cooking oil, ethanol, liquefied petroleum gas, diesel fuel, jet fuel, and other petroleum distillates.
- To increase the octane rating, additional substances like branched and cyclic alkanes are added to the gasoline fraction that remains after crude oil distillation.
Why do we crack hydrocarbons?
A significant number of hydrocarbons are obtained through the distillation of crude oil. Fractional distillation is a process that yields several hydrocarbon fractions, each serving distinct purposes and having varying levels of demand.
- Hydrocarbons with shorter chains exhibit lower viscosity and boiling points. Shorter-chain hydrocarbons are in higher economic demand due to their greater usefulness compared to longer-chain hydrocarbons. Naphtha, which is a fraction containing hydrocarbons ranging from C6 to C12, is currently experiencing a high demand. Petrol and the chemical industry both utilize it.
- Hydrocarbons with longer chains exhibit higher viscosity and boiling points. Shorter-chain hydrocarbons are more useful and therefore have a higher economic demand compared to longer-chain hydrocarbons. Longer-chain alkanes are too viscous to be utilized as gasoline, hence they are employed in aircraft instead.
- The demand for various crude oil fractions exceeds their supply, which poses a problem for their practical utility. An instance of this is that the crude oil sourced from the North Sea generally comprises more than 88% of long-chain C10+ hydrocarbons. We are left with a significant amount of longer-chain hydrocarbons that we have no practical use for.
- Hydrocarbons belonging to the C10+ category consist of chains that are comprised of a minimum of ten carbon atoms. Naphtha contains hydrocarbons ranging from C6 to C12, which means that the chains of these hydrocarbons consist of carbon atoms that are between six and twelve in number.
The SUPPLY and DEMAND for various fractions of crude oil
| .A comparison of typical percentages we get, and what we want, from the fractions of crude oil | |||
| Crude oil fraction | Carbon atoms in hydrocarbon | Approximate % in crude oil | Approximate % required |
| Gas, LPG etc. | 1 – 4 | 2-4 | 4 |
| Petrol-gasoline | 5-10 | 6-13 | 20-26 |
| Naphtha | 7-14 | 10-12 | 5 |
| Paraffin-kerosene | 9-16 | 12-15 | 8 |
| Diesel-gas oil | 15-19 | 13-20 | 20-25 |
| Mineral oils fuel/heavy oils, waxes, bitumen etc. | 20-40+ | 40-50 | 38 |
Cracking longer-chain fractions into more useful products can increase their economic value. Cracking is an economically significant reaction because the hydrocarbons produced are in higher demand and more useful to us than the original longer-chain hydrocarbons.
Applications of cracking
The cracking is relevant in the following applications.
Gasoline preparation (automotive gasoline): Cracking produces more than 50% of the fuel. In terms of anti-knocking properties, it provides gasoline of higher quality.
Preparation of gas and oil: Methane, ethane, propane, butane, and extremely minute amounts of other hydrocarbons are all present in oil gas, along with hydrogen. Kerosene oil is cracked by dropping it over a red-hot iron retort to produce oil gas.
Polymerization: To create polymers, simple alkenes from the pyrolysis process are used. These polymers may be petroleum-based.
Real-World Example of Cracking
While cracking is a prevalent step in the oil refining procedure, certain varieties of oil, such as light sweet crude oil, necessitate minimal processing before they can be marketed. Due to their low initial investment requirements before being sold, these particular types of oil are in great demand and can fetch premium prices on global commodities exchanges.
Whilst crude oil can be refined into numerous products, heating oil and gasoline are the most frequently traded commodities on the market. The ratio between two commodities is subject to temporal fluctuations based on the principles of supply and demand. However, traders often employ a heuristic that suggests the ratio between the two commodities should ideally hover around 3 to 2 to 1. Stated differently, this ratio postulates that a standard conversion of three barrels of oil would result in the production of two barrels of gasoline and one barrel of heating oil.
In instances where prices significantly deviate from these ratios, traders may engage in speculation with the aim of reverting to the mean. This may involve purchasing the commodity that appears undervalued in relation to the ratio or selling the one that appears overvalued. This ratio may serve as a useful reference point for traders who aim to mitigate their exposure to said commodities through hedging strategies.
References
- https://collegedunia.com/exams/cracking-science-articleid-721
- https://www.britannica.com/technology/cracking-chemical-process
- https://www.bbc.co.uk/bitesize/guides/zshvw6f/revision/5
- https://byjus.com/chemistry/cracking-meaning/
- Aly A Hamouda, Sidra Chughtai, ‘Miscible CO2 Flooding for EOR in the Presence of Natural Gas Components in Displacing and Displaced Fluids’, Energies 11(2):391 (February 2018)
- https://intotheoutdoors.org/segments/crude-oil-cracking-environmental-concerns-2/
- Alfke, Gunter; Irion, Walther W.; Neuwirth, Otto S. (2007). “Oil Refining”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_051.pub2.
- https://testbook.com/chemistry/cracking-meaning
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/cracking-chemistry/
- https://studymind.co.uk/notes/methods-of-cracking/
