Interesting Science Videos
Covalent bond Definition
A covalent bond is a type of chemical bonding resulting from the mutual sharing of electrons between two atoms of the same or different elements.
- The bond is the electrostatic interaction between the electrons present in the orbit of one atom and the protons present in the nucleus of the other atom.
- The number of electrons that are shared between two atoms in a covalent bond is called the covalency of the atoms.
- The purpose of covalent bonding is to attain a stable electronic configuration by both the atoms involved.
- Covalent bonding also contains other forms of interaction between the atoms like -bonding, -bonding, three-center two electrons bonds, and three-center four electrons bonds.
- The formation of a covalent bond, like all other bonds, release energy which decreases the total energy of the system. The total energy of the resulting compound should be lesser than that of the separated atoms for the covalent bonds to form.
- Covalent bonding was first introduced by the American chemist G.N. Lewis in 1916.
- A covalent bond is unique in that it can exist between two atoms of the same element, resulting in the molecular form.
- Covalent bonding between carbon atoms is the basis of organic chemistry as it enables the formation of thousands of organic compounds found in various living systems.
- In some organic compounds, the electrons shared in a covalent bond can be localized between multiple atoms in order to achieve a stable configuration.
- Covalent compounds usually have a lower boiling and melting point due to the lesser strength of the bond when compared to other bonds like ionic bonds.
- The covalent bond is directional in nature due to the overlapping of orbitals that are oriented in different directions. The direction of the bond depends on the direction of the orbitals involved in the bond.
- Covalent bonds often have a low polarity as the electrons are shared among the atoms, causing the spreading of the charges.
- Covalent bonding is usually observed between atoms with a lower difference in electronegativity.
Ionic Bond Definition
The ionic bond is an electrostatic interaction between two oppositely charged atoms or ions with different electronegativities as a result of the transfer of electrons from one chemical species to another.
- During the ionic bonds, charged ions are formed due to the transfer of electrons from one atom to another. The atom that loses electrons becomes a positively charged ion called a cation, whereas the atom that gains electrons becomes a negatively charged ion called an anion.
- Ionic bonds are usually formed between metals and nonmetals, where the metal ions act as cations and the nonmetal ions act as anions.
- Ionic bonding is the result of electrostatic forces of attraction and repulsion between opposite charges and similar charges, respectively.
- The compounds formed as a result of ionic bonding are called ionic compounds.
- Ionic interactions or electrovalent interactions are usually observed in neutralization reactions between acids and bases. Thus, most ionic compounds are salts.
- Ionic bonds are not always pure as all ionic compounds have some degree of covalent binding as a result of electron sharing. The term ionic is used when the ionic character of the bond is greater than the covalent character.
- The number of electrons transferred between two atoms involved in the bond is known as the electrovalency of the atoms.
- The strength of the ionic bond depends on the number of electrons shared between the atoms, which, in turn, depends on the electronic configuration of the atoms.
- Ionic bonds are non-directional as the force of attraction between the oppositely charged ions acts equally in all directions.
- An ionic bond is responsible for the electrical conductivity of compounds as the atoms involved in ionic bonding can ionize into ions that can then conduct electricity.
- Since the force of attraction between the atoms in ionic compounds is stronger, ionic compounds usually have higher boiling and melting points.
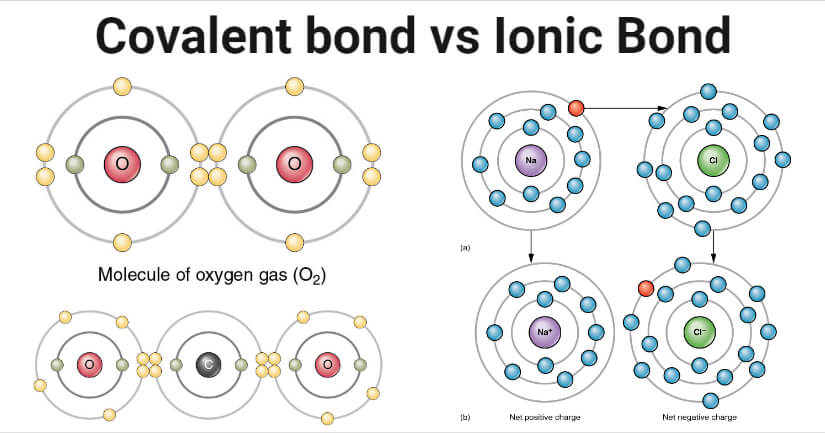
Image Source: OpenStax.
11 Major Differences (Covalent vs Ionic Bond)
| Characteristics | Covalent Bond | Ionic Bond |
| Definition | A covalent bond is a type of chemical bonding resulting from the mutual sharing of electrons between two atoms of the same or different elements. | The ionic bond is an electrostatic interaction between two oppositely charged atoms or ions with different electronegativities as a result of the transfer of electrons from one chemical species to another. |
| Strength | Covalent bonds are comparatively weaker than ionic bonds. | Ionic bonds are relatively stronger than covalent bonds. |
| Atoms involved | Covalent bonds usually form between nonmetals with a lower difference in electronegativity. | Ionic bonds usually form between metals and nonmetals. |
| Formation of the bond | Covalent bonds are formed as a result of the sharing of electrons. | Ionic bonds are formed as a result of the transfer of electrons. |
| Compounds | Compounds formed by covalent bonding are called covalent compounds. | Compounds formed by ionic bonds are called ionic compounds. |
| Covalent compounds usually have lower boiling and melting points. | Ionic compounds usually have higher melting and boiling points. | |
| Covalent compounds exist as molecules (liquid or gas) at room temperature. | Ionic compounds exist in the form of a crystal (solid) at room temperature. | |
| Covalent compounds cannot conduct electricity. | Ionic compounds can conduct electricity. | |
| Polarity | Covalent bonds are directional in nature. | Ionic bonds are non-directional. |
| Difference in electronegativity | The difference in the electronegativity values of the atoms should be lesser than 1.7. | The difference in the electronegativity values should be higher than 1.7. |
| Examples | Examples of covalent bonds can be seen in molecules like O2, CO2, N2, etc. | Examples of ionic bonding can be seen in molecules like NaCl, MgSO4, etc. |
Examples of Covalent bond
Covalent bonding in O2
- O2 is the molecular form of the oxygen atom as oxygen is a diatomic gas. The bond between the two oxygen atoms in the molecule is covalent.
- Two oxygen atoms in an oxygen molecule are bonded by double covalent bonds where each atom shares a pair of electrons.
- The covalent bond oxygen molecule is not polar as there is no difference in electronegativity between the two atoms. The bond is formed as a result of the true sharing of electrons between the two atoms.
- The covalent compound thus formed exists in a gaseous state as the bond is weaker than polar covalent bonds or ionic bonding.
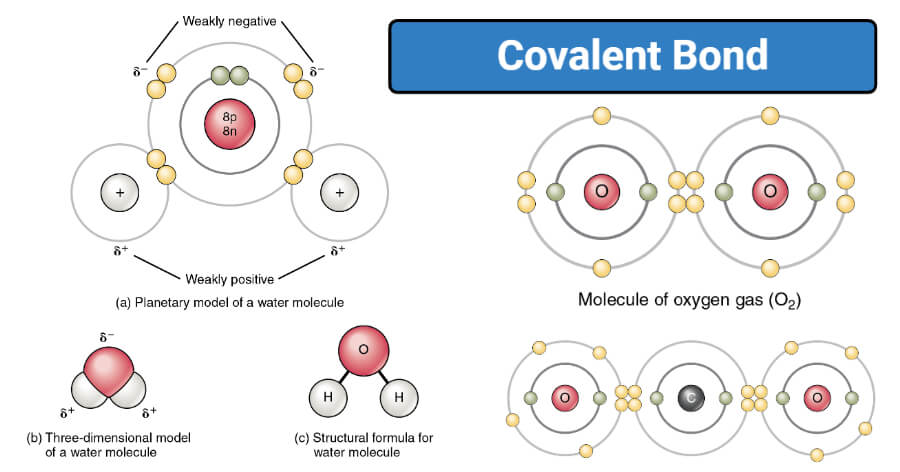
Image Source: OpenStax.
Examples of Ionic bond
Ionic bonding in NaCl
- The bond between the sodium atom (Na) and the chlorine atom (Cl) in sodium chloride (NaCl) is ionic, and NaCl is an ionic compound.
- The sodium atom has one electron in its outermost shell, indicating its ability to attain a stable electronic configuration by losing the electron.
- Chlorine, on the other hand, has seven electrons in its outermost shell and requires one electron to complete the stable configuration.
- During the formation of the bond, the sodium atom loses one electron, resulting in the formation of sodium cation, and the chlorine atom accepts the electron to form the chloride anion.
- The ionic nature of the bond can be observed via the physical properties of the compound, like crystalline structure and high melting point.
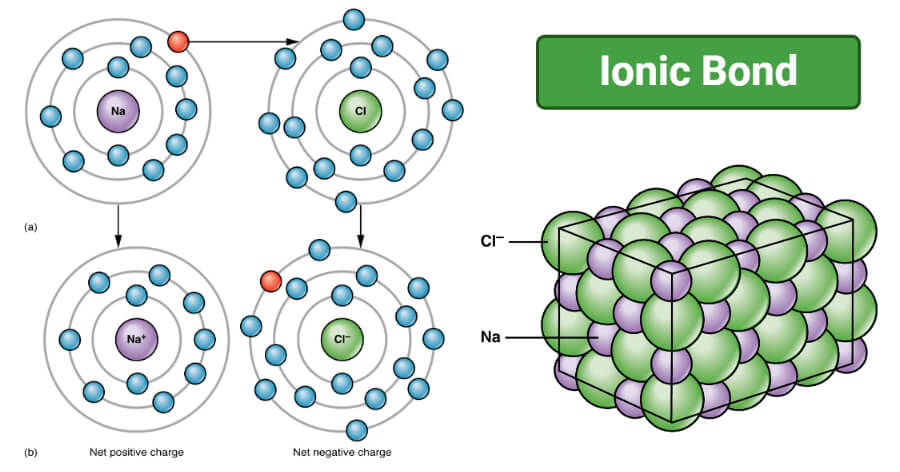
Image Source: OpenStax.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- Robert J. Ouellette, J. David Rawn. Structure of Organic Compounds. Principles of Organic Chemistry, Elsevier. 2015. Pages 1-32. https://doi.org/10.1016/B978-0-12-802444-7.00001-X.
- 2% – https://chem-guide.blogspot.com/2010/04/ionic-bonds-ionic-compounds-and.html
- 2% – https://byjus.com/chemistry/formation-of-ionic-compounds/
- 1% – https://www.thefreedictionary.com/covalent+bond
- 1% – https://www.icserankers.com/2020/11/icse-solutions-for-chapter2-chemical-bonding-class10-chemistry.html
- 1% – https://quizlet.com/558786088/ionic-bonding-flash-cards/
- 1% – https://quizlet.com/391915085/ch-9-learnsmart-flash-cards/
- 1% – https://findanyanswer.com/which-isomer-of-hexane-has-the-highest-boiling-point
- 1% – https://brainly.in/question/19952186
- 1% – https://biologydictionary.net/ionic-bond-examples/
- 1% – https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Boundless)/2%3A_Chemistry/2.2%3A_Chemical_Bonds/2.2.2%3A_Colvalent_Bonds_and_Other_Bonds_and_Interaction
- 1% – https://bio.libretexts.org/Bookshelves/Human_Biology/Book%3A_Human_Biology_(Wakim_and_Grewal)/03%3A_Chemistry_of_Life/3.03%3A_Chemical_Bonding
- 1% – https://answers.yahoo.com/question/index?qid=20100119193607AAh72TA
- <1% – https://www.vedantu.com/iit-jee/covalent-bond
- <1% – https://www.orangatame.com/polar-covalent-bold/
- <1% – https://www.encyclopedia.com/science/encyclopedias-almanacs-transcripts-and-maps/chemical-bonds-and-physical-properties
- <1% – https://www.britannica.com/science/valence-chemistry
- <1% – https://www.bbc.co.uk/bitesize/guides/zy98msg/revision/4
- <1% – https://www.aplustopper.com/properties-ionic-covalent-compounds/
- <1% – https://vivadifferences.com/difference-between-covalent-and-ionic-bonds-with-similarities/
- <1% – https://sciencing.com/do-compounds-conduct-electricity-water-6681297.html
- <1% – https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/
- <1% – https://mikeblaber.org/oldwine/chm1045/notes/Bonding/Polarity/Bond05.htm
- <1% – https://frontline.thehindu.com/science-and-technology/article30182589.ece#!
- <1% – https://courses.lumenlearning.com/trident-boundless-chemistry/chapter/the-ionic-bond/
- <1% – https://courses.lumenlearning.com/cheminter/chapter/energy-and-covalent-bond-formation/
- <1% – https://chemistry.stackexchange.com/questions/6732/is-ionic-bond-just-formed-by-electrostatic-interaction-between-two-oppositely
- <1% – https://byjus.com/jee/sigma-and-pi-bond/
- <1% – http://www.physics.smu.edu/scalise/P6338sp16/gonul/chapter2.pdf
- <1% – http://isch.skhsslmc.edu.hk/~chem/AL%20Notes/Unit%204%20Bonding.pdf
