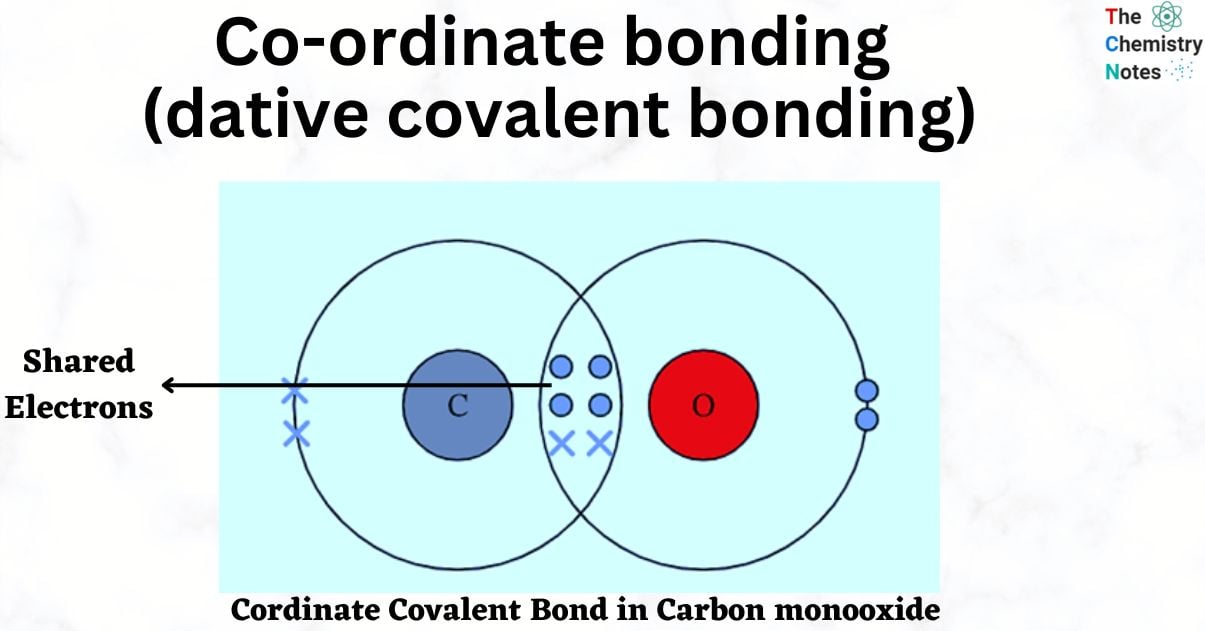
A coordinate covalent bond, also known as a dative bond, is a type of covalent bond in which both electrons originate from the same atom. It typically occurs in molecules where metallic ions are bonded to ligands. When two nonmetal atoms share a pair of electrons, covalent bonds form. The atom that donates the lone pair of electrons is referred to as the donor atom, and it is classified as a Lewis base. The acceptor atom is a Lewis acid that accepts the lone pair of electrons into a vacant orbital.
Interesting Science Videos
What is Coordinate Covalent Bond?
A coordinate covalent bond, also known as a dative bond or coordinate bond, is a type of covalent bond that forms when both shared bonding electrons come from only one of the atoms.
The broader term “covalent bond” refers to conditions in which each atom contributes a bonding electron or where one atom contributes both electrons. The attraction of both nuclei to the electron pair holds a coordinate covalent bond together. Coordinate covalent bonds form in Lewis acid and base reactions, when metal ions bond to ligands, and occasionally when nonmetals bond to metals.
In order to form dative covalent bonds, we need:
- one atom with a lone pair of electrons
- a second atom having an unfilled orbital to accept the lone pair; in other words, an electron-deficient compound
Coordinate Covalent Bond Characteristics
- The atom that shares an electron pair with itself is referred to as the donor in this type of bonding.
- A receptor or acceptor is the other atom that accepts this shared pair of electrons.
- The bond is represented by an arrow pointing from the donor atom to the acceptor.
- Each atom gains stability after sharing electron pain.
- The Lewis theory relies heavily on this type of bonding.
- Understanding co-ordinate covalent bonds can aid in the proper design of complex organic molecules.
- The bond is rigid and directional.
- Compounds formed are sparingly soluble in water.
Examples of Coordinate Covalent Bond
Ammonium Ion (NH4+)
Ammonia molecules are polyatomic molecules with one nitrogen atom and three hydrogen atoms that are neutrally charged. The nitrogen atom has five valence electrons, three of which are used to form covalent bonds with the three hydrogen atoms. The final two valence electrons are not bonded to any atoms. The final two valence electrons exist as a single pair and can form a coordinate covalent bond with another acceptor atom.
The ammonium ion, NH4+, is formed when ammonia combines with a hydrogen ion, H+. The hydrogen ion is electron-deficient, with only two electrons available in its shell. In the ammonia molecule, the nitrogen atom has a single pair of electrons. The nitrogen atom’s lone pair provides both electrons for the bond.
In a displayed formula (which shows all atoms and bonds), a coordinate bond is represented by an arrow. The arrow’s head is pointing away from the bond’s lone pair.
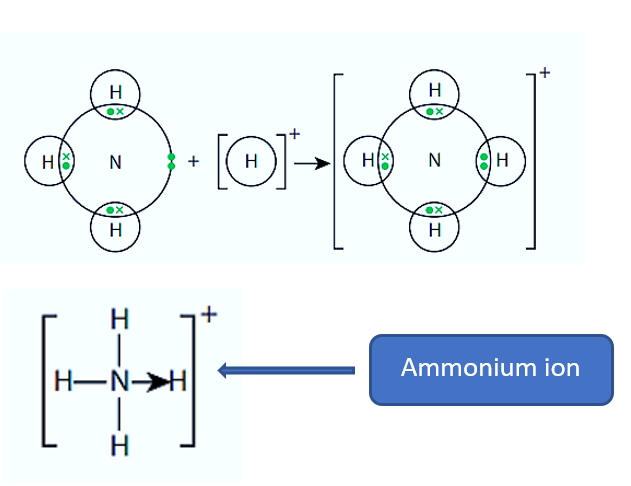
ammonium ion
Aluminum Chloride (Al2Cl6)
Aluminum chloride (AlCl3) bonds are essentially covalent. Each aluminum (Al) atom has two electrons missing from its valence shell, while chlorine (Cl) has a single pair. The Al atom forms a covalent bond with the Cl atom on an adjacent AlCl3 group. Aluminum chloride is a covalent dimer molecule with the formula Al2Cl6 because each of two Al atoms does this.
Aluminium Chloride molecules with the formula AlCl3 exist at high temperatures. This molecule is electron-deficient; it still requires two electrons to complete the aluminum atom’s outer shell. Lone pairs of electrons on two of the chlorine atoms form coordinate bonds with the aluminum atoms, allowing the AlCl3 molecules to combine.
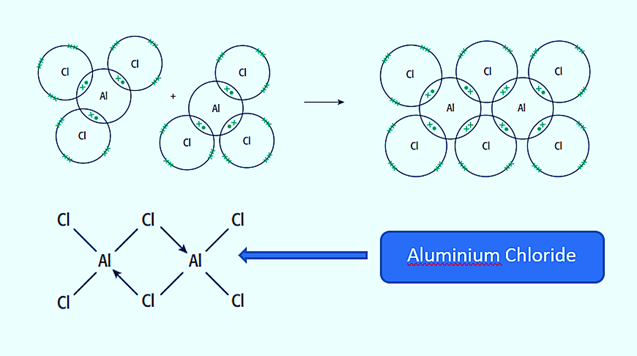
Aluminium chloride
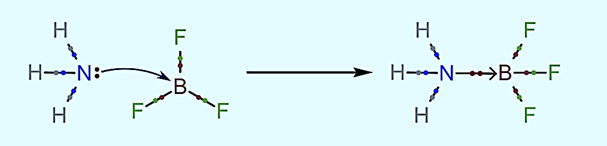
Ammonia and Boron Trifluoride (H3N→BF3)
Ammonia is a Lewis base because it has a donatable electron pair. Boron trifluoride has one empty orbital that can accept an electron pair from ammonia to complete its valence shell, allowing it to act as a Lewis acid. Because it has equal negative and positive charges, the ammonia-boron trifluoride adduct is neutral.
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN: 978-0-08-037941-8.
- Haaland, Arne (1989). “Covalent versus Dative Bonds to Main Group Metals, a Useful Distinction”. Angewandte Chemie International Edition in English. 28 (8): 992–1007. doi:10.1002/anie.198909921
- https://sciencenotes.org/coordinate-covalent-bond-dative-bond/
- https://goldbook.iupac.org/terms/view/D01752
- https://www.nagwa.com/en/explainers/235126567926/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/ Coordinate_ (Dative_Covalent)
- _Bondingmel, Daniel; Krossing, Ingo; Schnepf, Andreas (2014). “Dative Bonds in Main-Group Compounds: A Case for Fewer Arrows!”. Angewandte Chemie International Edition. 53 (2): 370–374. doi:10.1002/anie.201300461
- https://byjus.com/jee/co-ordinate-bond/
