The combined gas law is a combination of three gas laws: Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law. It states that the ratio between the product of a gas’s pressure and volume and its absolute temperature is constant. The ideal gas law is obtained by combining Avogadro’s law with the combined gas law. The combined gas law does not have a specific discoverer, unlike the named gas laws. It is a combination of the other gas laws that only consider temperature, pressure, and volume when everything else is constant.
Interesting Science Videos
What is Combined Gas Law?
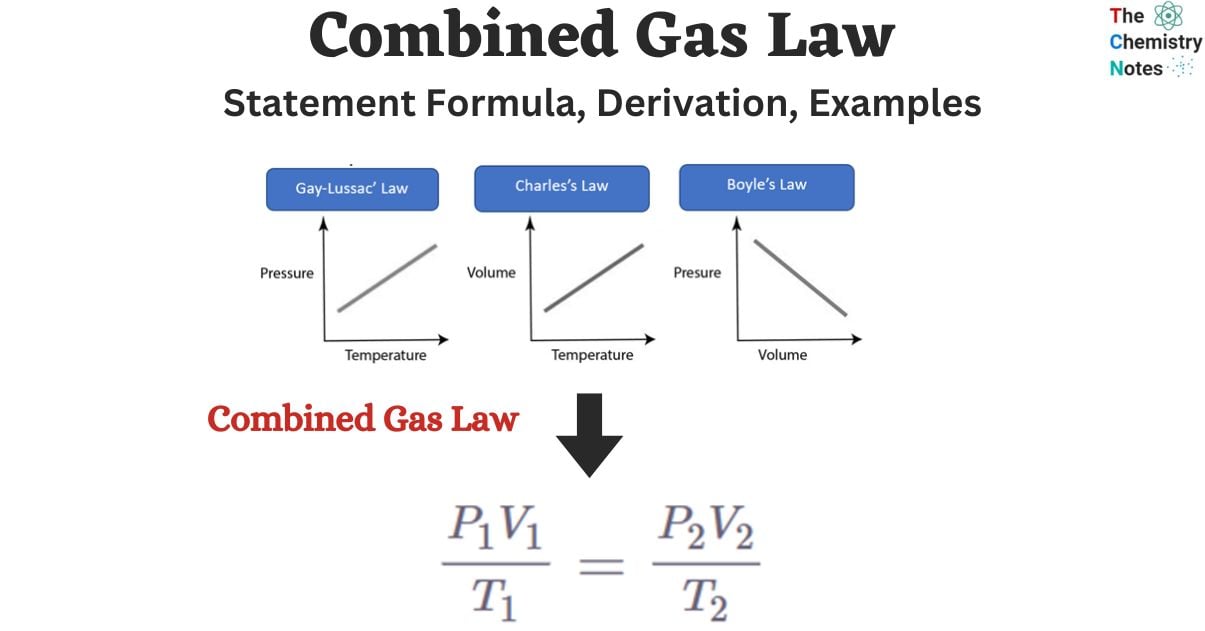
The Combined Gas Law states that when the amount of gas is fixed, the product of pressure (P), volume (V), and temperature (T) is equal to a constant (k).
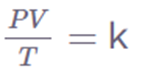
The combined gas law is formed by combining four different laws.
Charles’ law states that the volume of a gas is directly proportional to its temperature, assuming the pressure and amount of gas remain constant.
Gay-Lussac’s law is a scientific principle that describes the relationship between the temperature and pressure of a gas.
Avogadro’s law refers to the relationship between the volume of a gas and the number of particles it contains.
Boyle’s law is a principle in physics that describes the relationship between the pressure and volume of a gas.
These laws relate one thermodynamic variable to another while keeping all other variables constant. The relationship between these variables is shown by the combined gas law. It states that the product of a system’s pressure, volume, and temperature remains constant.
The Combined Gas Law is written as:
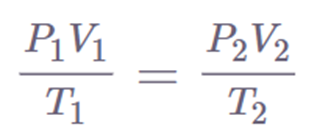
Combined Gas Law Formula
Combined gas law can be mathematically expressed as
k = PV/T
Where,
P = pressure
T = temperature in kelvin
V = volume
K = constant
In two different conditions, the law can be stated as,
Pi Vi/ Ti = Pf Vf / Tf
Where,
Pi = initial pressure
Vi = initial volume
Ti = initial temperature
Pf = final pressure
Vf= final volume
Tf = final temperature
Derivation of the Combined Gas Law
The combined gas law is derived from combining Charles’ Law, Boyle’s Law, and Gay-Lussac’s Law.
When we combine all these relationships, we obtain the combined gas law equation.
The ideal gas law is obtained by expanding the combined gas law when the number of gas moles (n) is not kept constant. You can derive the other gas laws by holding different variables constant and working backward from the ideal gas law. In the combined gas law, the moles of gas (n) would be kept constant.
Example Problem: Combined Gas Law
Determine the volume of a gas under the conditions of 760.0 mm Hg pressure and 273 K temperature, given that 2.00 liters of the gas were collected at 745.0 mm Hg pressure and 25.0 °C temperature.
Solution:
To convert a temperature from Celsius to Kelvin, one must add 273.15 to the given temperature. Therefore, to convert 25.0 °C to the Kelvin scale, one would add 273.15 to obtain the equivalent temperature in Kelvin. This results in a temperature of 298 Kelvin.
P1 = 745.0 mm Hg
V1 = 2.00 L
T1 = 298 K
P2 = 760.0 mm Hg
V2 = x
T2 = 273 K
Arrange the formula
P1V1 / T1 = P2V2 / T2
P1V1T2 = P2V2T1
V2 = (P1V1T2) / (P2T1)
Now, substituting the Values;
V2 = (745.0 mm Hg · 2.00 L · 273 K) / (760 mm Hg · 298 K)
V2 = 1.796 L
V2 = 1.80 L
References
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry. Holt, Rinehart and Winston. ISBN 978-0-03-056537-3.
- Raff, Lionel M. (2001) Principles of Physical Chemistry (1st ed.). Pearson College Div. ISBN: 978-0130278050.
- Helmenstine, Anne Marie, Ph.D. “The Combined Gas Law in Chemistry.” ThoughtCo, Nov. 19, 2022, thoughtco.com/definition-of-combined-gas-law-604936.
- https://byjus.com/combined-gas-law-formula/
- https://chemistrytalk.org/combined-gas-law-chemistry/
- https://www.expii.com/t/combined-gas-law-overview-calculations-11097
- https://collegedunia.com/exams/combined-gas-law-formula-evolution-and-solved-examples-chemistry-articleid-1812
