The colorimeter is an optical apparatus that has been specifically engineered to detect and measure the absorption of distinct wavelengths of light across a range of solutions.
By virtue of its sensitivity to light, this device enables researchers to investigate the optical properties of substances and gain insights into their molecular composition and behavior. The spectrophotometer, a widely employed scientific instrument, serves the purpose of quantifying the absorbance and transmittance of light as it traverses a liquid sample.
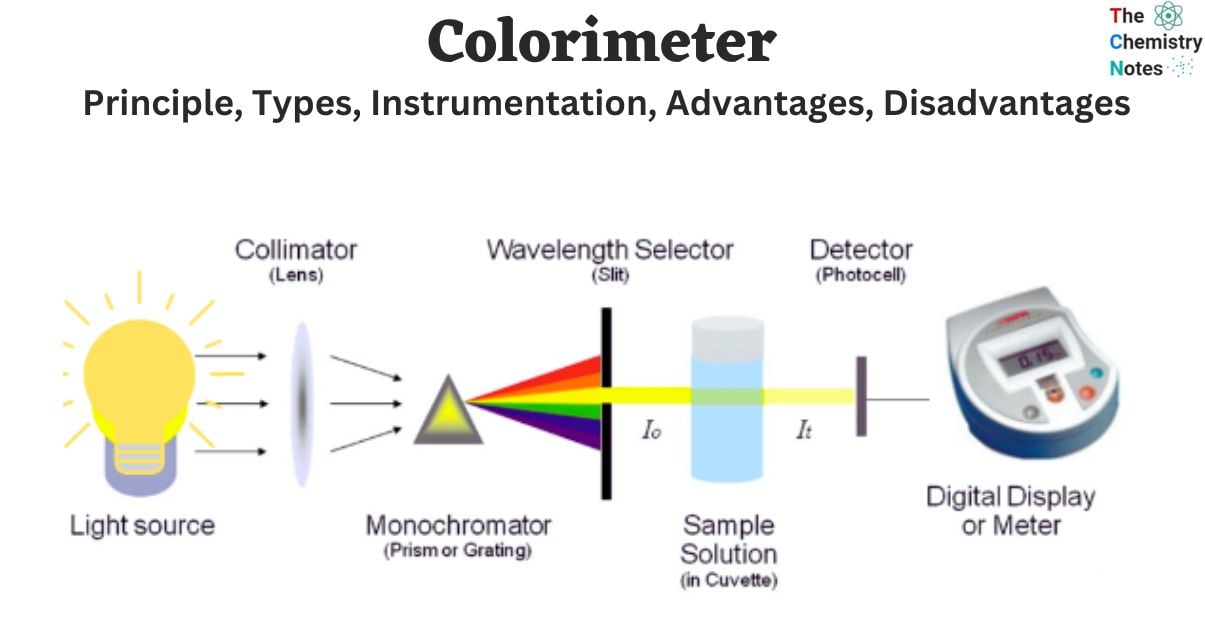
The colorimeter apparatus possesses the ability to quantitatively assess the intensity or concentration of color that emerges upon the introduction of a particular reagent into a solution. The operational capabilities of a colorimeter are deeply grounded in the fundamental principle commonly referred to as Beer-Lambert’s law. The colorimeter, a seminal scientific apparatus, was devised by the esteemed inventor Louis J. Duboscq in 1870.
Types of Colorimeter
Spectrophotometer
- The spectrophotometer is a sophisticated and precise scientific instrument utilized for the purpose of quantifying the spectral reflectance of a given surface.
- This particular device is considered to be the most precise version of a colorimeter, as it provides advanced functionalities for the measurement and analysis of the distribution of wavelengths in reflected light.
Densitometer
- A densitometer is a highly specialized instrument that is utilized to measure and quantify the density of a particular substance.
Tristimulus Colorimeter
- The tristimulus colorimeter is a commonly employed instrument used to measure and quantify the tristimulus values that correspond to a specific color.
Principles of Colorimeter
- The underlying principle of a colorimeter revolves around the quantification of light absorption exhibited by a given sample solution.
- When a beam of incident light, characterized by its intensity (I0), traverses a solution, it experiences three distinct phenomena: reflection (Ir), absorption (Ia), and transmission (It).
The various constituents of light can be mathematically represented in the following manner:
Io = Ir + Ia + It- The colorimeter is employed to quantify the absorption of light (Ia) by effectively excluding the influence of reflected light (Ir).
- By employing cells that possess consistent characteristics, it is possible to maintain a constant level of light reflection.
- The colorimeter is a device used to quantify the intensity of incident light (I0) and transmitted light (It).
The functioning of a colorimeter relies on the principles of Beer-Lambert’s law, a fundamental concept in spectroscopy. - According to this law, the degree of light absorption exhibited by a colored solution is directly correlated to both the concentration of the solution and the distance that the light traverses through it.
In a mathematical context, this particular association can be formally represented as follows:
- A ∝ cl
- A = ∈cl
- Where,
- A = Absorbance/ Optical density of the solution
- ∈ = Coefficient of absorption
- c = Concentration of solution
- l = Length of the path

[Image: Beley.com]
Instrumentation of Colorimeter
Light
- The light source employed should have the capacity to generate a sufficient amount of energy throughout the entire range of wavelengths that are visible to the human eye, ranging from (380-780 nm).
- Tungsten lamps are extensively employed in a diverse range of applications.
- Halogen deuterium is suitable for measurement in the UV range (200-900 nm).
Slit
- A slit is a device that is specifically engineered to allow the controlled passage of a beam of light while simultaneously minimizing the interference caused by unwanted ambient light.
Condensing Lens
- A condensing lens is employed to produce a collimated light beam.
Monochromator
- A monochromator is an apparatus designed to convert polychromatic radiation, such as white light, emitted from a light source into monochromatic radiation that is composed of a single wavelength band.
- This phenomenon facilitates the propagation of the particular wavelength that is required.
- A range of optical devices can be employed for this objective that includes
- Prisms: Prisms possess the ability to effectively disperse white light into its individual constituent wavelengths. The desired spectrum is selected by rotating the prism. The colorimeter’s prism is constructed from glass and operates effectively within the wavelength range spanning from 350-800 nm.
- Grating Monochromators: A grating monochromator is a device that produces monochromatic light by utilizing a polished surface made of steel, glass, or quartz with closely spaced parallel grooves. A typical grating is characterized by a line density ranging from 500-600 lines/mm, while advanced research equipment often exhibits a higher line density of 1200- 2000 lines/mm. When white light interacts with a grating, it undergoes a phenomenon known as dispersion, where the various wavelengths of light are separated and deflected at different angles.
- Interference Filters: An interference filter is comprised of multiple silver sheets that exhibit both semi-transmissive and semi-reflective properties. The sheets are divided by thin layers of transparent dielectric material. When white light traverses the dielectric layers, it undergoes multiple reflections between the semitransparent mirrors. In this particular scenario, a fraction of the energy carried by the light ray is capable of traversing the filter without experiencing any deviation or obstruction. The determination of the required wavelength for analysis is conducted. The wavelength of light is determined by the thickness of the dielectric layer.
- Gelatin Filters: Gelatin filters consist of a thin layer of colored gelatin that is sandwiched between two fragile glass plates. The cost-effectiveness of these filters is noteworthy; however, it is crucial to take into account their ability to absorb a substantial percentage, approximately 30-40%, of incoming radiation. As a result, this absorption may lead to a decrease in energy throughput for the detectors.
- Glass: Tinted glass filters are a specific type of filter that provide an alternative option due to their wide bandpasses, which can reach up to 150 nm. The attainment of specific wavelengths can be facilitated by combining multiple glass filters.
Sample Holder (Cuvette)
- The monochromatic light emitted by the filter passes through the cuvette containing the solution, which displays a wide spectrum of colors.
- Cuvette exhibit a range of shapes, including squares, rectangles, and circles, all of which possess a uniform diameter of 1cm.
- There are three distinct categories of cuvettes: glass, quartz, and plastic cuvettes. These categories are determined by the composition of the cuvettes.
- Glass Cuvettes: These are a cost-effective option for light absorption at a specific wavelength of 340 nm.
- Quartz Cuvettes: It possess the ability to transmit both ultraviolet (UV) and visible light.
- Plastic Cuvettes: They are characterized by their lower cost, increased susceptibility to scratching, and relatively shorter lifespan.
Photo Detectors
- Photodetectors play a crucial role in converting the transmitted light rays into an electrical signal after they have traversed the sample container.
- This device is commonly referred to as a photocell. Colorimeters employ various types of sensors depending on the material used.
- Commonly utilized detectors include Selenium (Se)-based photocells, phototubes, and silicon-based photocells.
- Selenium Photocell: It is a basic type of detector that functions autonomously, without reliance on external power sources.
- Phototube: It is a device that comprises a glass bulb that has been coated with photosensitive substances, such as cesium or potassium.
- Silicon Photocell: It functions by producing electrons when a photon of light interacts with its semiconductive surface.
Filter
- The choice of filters depends on the particular manufacturer of the colorimeter.
- Light can be categorized into two main types:
- Monochromatic: Monochromatic light is characterized by a single wavelength.
- Polychromatic: polychromatic light consists of multiple wavelengths.
- The categorization of light into monochromatic or polychromatic is based on its wavelength.
- White light, for instance, is considered polychromatic because it consists of a combination of multiple wavelengths.
Galvanator
- The device is capable of monitoring and quantifying electrical signals, subsequently generating a visible output.
- The galvanometer is responsible for detecting and measuring the electrical signal produced within a photocell.
- The device exhibits measurements of optical density (OD) and percentage transmission.
How To Operate Colorimeter
Step 1
- To initiate the colorimeter, please activate the device by turning the Power Switch knob in a clockwise direction. It is recommended to allow the colorimeter to warm up for approximately 15 minutes in order to ensure the stabilization of both the light source and detector.
Step 2
- After the completion of the warm-up period, proceed to adjust the Wavelength Control knob to the preferred wavelength for the intended measurement.
Step 3
- To activate the transmittance display mode, please press the MODE control key until the indicator light next to “Transmittance” is illuminated.
Step 4
- Please adjust the T-factor display to 0.0% by utilizing the Zero Control knob. Please make sure that the sample chamber is devoid of any contents and that the cover is firmly closed while performing this adjustment.
Step 5
- Please prepare a blank solution by carefully filling a cuvette with the solution until it reaches the top of the triangle marking on the side. Please use a clean cloth or Kimwipe to gently clean the exterior of the cuvette. This will help eliminate any fluids or fingerprints that may obstruct light transmission and potentially impact the accuracy of readings.
Step 6
- Carefully and thoroughly place the cuvette into the sample chamber, ensuring that the vertical guideline on the cuvette is properly aligned with the guideline on the sample chamber. To prevent any potential scratches on the light-transmitting parts, it is advisable to rotate the cuvette in a clockwise direction by 90 degrees. Please close the compartment cover.
Step 7
- Adjust the Transmittance/Absorbance control knob to achieve a display setting of 100.0%.
Step 8
- To change the Status Indicator light to display Absorbance, please press the MODE control key. If the Transmittance calibration has been performed accurately, the display should indicate a value of 0.0. If the display does not show a value of 0.0, the Transmittance/Absorbance control knob can be used to make the necessary adjustment. To revert the display to Transmittance, please utilize the MODE key.
Step 9
- Reverse the previous process to remove the cuvette from the compartment by rotating it 90 degrees counter-clockwise before doing so. You should put the solution whose absorbance you want to test in another cuvette. Similar to before, place it inside the chamber.
- (a) Directly from the digital display, read the %T value.
- (b) Select Absorbance using the MODE key, then take the A value directly from the digital display. Select Transmittance once more.
Step 10
- Reverse the process you used to insert the cuvette to remove it from the sample compartment. Close the compartment’s cover.
Difference Between Spectrophotometer and Colorimeter
| Properties | Colorimeter | Spectrophotometer |
|---|---|---|
| Function | The main purpose of a colorimeter is to gauge how much transmitted light a particular solution absorbs | Spectrophotometer evaluates the intensity of light as a function of the color or wavelength of light by measuring the transmittance level. |
| Approach | The colorimeter results in psychophysical analysis, providing color measurement physiologically similar to how the human eye and brain perceive color. | As a result of the spectrophotometer’s physical analysis, colorimetric information can be collected indirectly. |
| Sensitivity | Colorimeters are regarded as less sensitive equipment. | Spectrophotometers are regarded much sensitive. |
| Cost | Colorimetres are cheaper. | Spectrophotometers are expensive. |
| Complexity | The colorimeter is less complex, lighter in weight, and more durable. | Spectrophotometer is considerably heavier and hence more complex. |
| Capability | Ability to immediately read colorimetric data and provide tristimulus values such as XYZ, G, b, d, etc | Spectrophotometers are capable of indirectly calculating psychophysical data, |
| Wavelength | The visible portion of the electromagnetic spectrum is the only light that the colorimeter can detect. | Spectrophotometers also measure invisible and ultraviolet light in addition to visible light. |
| Weight | Colorimeters are lighter. | Spectrophotometers are heavier. |
| Display of Data | Colorimeters display data on a digital or analog output. | Spectrophotometers generate and record data using software. |
| Application | On the basis of absorbance, colorimeters can be used to determine the concentration of an individual component. | Spectrophotometers can be used to identify and quantify inorganic and organic biological compounds. |
How Colorimeters Analyze Color
- To analyze the concentration of a mysterious sample, numerous specimens are carefully made from the test sample and then examined using a very advanced colorimeter.
- Upon careful examination, the transmittance and concentration values of the specimen under investigation are meticulously plotted on a graph, thereby yielding a visual depiction of the concentration known as a calibration curve.
- Following this, the previously mentioned curve is compared with the curve obtained from a sample of recognized credibility, so enabling the measurement of concentration.
How to Maintain Colorimeter
Here are some considerations to keep in mind when maintaining a colorimeter:
- It is recommended that users consistently remove the cuvette from the instrument when it is not being used.
- If any optical marks are observed on the cuvette, it is advisable to gently clean them using tissue paper.
- It is recommended to power off the colorimeter when it is not in use. By implementing this approach, the longevity of the lamp can be enhanced, while also conserving energy.
- After completing the task, it is recommended to disconnect the plug from the switchboard and securely cover the colorimeter with its designated protective cover.
- It is recommended to establish a regular practice of inspecting the primary power adapter and cable for indications of damage or deterioration. The continued use of the colorimeter instrument, even when there are only minimal signs of deterioration, can pose risks of both machine impairment and potential harm.
- In the event that the instrument sustains damage, it should be replaced.
- It is advisable to store the item in cool environments and maintain it at room temperature.
- It is advisable to avoid storing it in close proximity to hazardous chemicals or combustible substances.
Applications of Colorimeter
Here are some of the important use of colorimeter:
- Colorimeters are widely utilized in chemistry laboratories for fundamental research purposes. Moreover, these instruments possess a multitude of practical applications, one of which involves assessing water quality by means of chemical screening. Various chemicals, such as fluoride, zinc, chlorine, cyanide, iron, dissolved oxygen, molybdenum, and hydrazine, can be effectively tested using colorimeters.
- Additionally, these instruments are utilized for the purpose of assessing the levels of plant nutrients, such as nitrate, ammonia, and phosphorus, present in the soil.
- Colorimeters are commonly employed for the purpose of analyzing color contrast and brightness in mobile, computer, and television screens, with the aim of enhancing the viewing experience for users.
- Colorimetry is a widely employed technique in various industries such as textile manufacturing, color printing, and paint manufacturing. It serves as a crucial tool for conducting meticulous quality inspections.
- This equipment is commonly utilized in laboratory and healthcare settings for the purpose of analyzing biochemical samples, including but not limited to urine, cerebrospinal fluid, plasma, and serum.
- This technique is employed in the quantitative analysis of proteins, glucose, and various other biochemical compounds.
- This tool is utilized for the purpose of assessing the quality of water.
- This method is employed to ascertain the concentration of hemoglobin in the bloodstream.
Advantages of Colorimeter
There are a lot of advantages of calorimeter some of which are listed here:
- The colorimeter is an inexpensive and highly effective tool for conducting quality analysis.
- Portable colorimeters are readily accessible, rendering them highly convenient for utilization.
- Colorimeters are equipped with a user-friendly interface that facilitates their ease of use and enables efficient data collection.
- The Colorimeter is a readily accessible tool that facilitates the quantitative analysis of colored compounds.
- Colorimeters are specifically designed for utilization within a controlled laboratory setting, thereby enabling the attainment of enhanced levels of measurement accuracy.
- Colorimeters are typically characterized by their low maintenance requirements, as they do not necessitate frequent calibration or other maintenance procedures.
Drawbacks of Colorimeter
Despite having a lot of advantages colorimeter also has some drawbacks which are listed here:
- The determination of the concentration of colorless substances can be a laborious and challenging process.
- The colorimeter has a restricted capability to measure the absorbance of wavelengths only within the visible range of light, which spans from 400nm to 700nm. It is not equipped to operate in the ultraviolet or infrared spectrum.
- To ensure precise measurement of absorbance, it is imperative to establish a range of wavelengths encompassing the entire spectrum, rather than relying solely on a single, specific wavelength.
- Obtaining precise measurements on surfaces with high reflectivity can pose certain challenges.
Frequently Asked Questions (FAQ)
What is the underlying principle of a colorimeter?
Colorimetry is a scientific discipline that involves the quantification of the concentration of a colored substance inside a given solution. A colorimeter is a scientific instrument utilized for quantitative analysis. It measures the absorption of light at a specific wavelength in order to determine the concentration of a solution.
What does a colorimeter measure?
A colorimeter is a device used to determine how much light is absorbed. The process of color measurement involves quantifying the alteration in the intensity of electromagnetic radiation within the visible wavelength range of the spectrum, subsequent to its transmission or reflection by an object or solution.
What is the operational range of the colorimeter?
The colorimeter functions within the visible range of the electromagnetic spectrum. That is to say, it has the capability to function across the range of wavelengths spanning from 400nm to 700nm. In the case of typical colorimeters, the available wavelength options are predetermined, allowing users to choose from a set range of wavelengths, specifically 430nm, 470nm, 565nm, and 635nm.
What distinguishes a colorimeter from a spectrophotometer?
Colorimeters are commonly designed to be portable in nature, employing light-emitting diode (LED) light sources and filters that manipulate color. Consequently, they function within specific wavelengths and are limited to conducting tests that are compatible with those wavelengths.
Spectrophotometers typically consist of tabletop instruments that employ light sources capable of generating a diverse array of wavelengths. In addition to their primary function, spectrophotometers employ monochromators to effectively isolate and choose the specific wavelength of interest. Consequently, spectrophotometers find utility in a diverse array of analytical procedures.
Video on Colorimeter
References
- https://www.hunterlab.com/blog/lab-vs-lch-coordinates/#:~:text=A%20colorimeter%20is%20a%20light,that%20passes%20through%20the%20object.
- https://byjus.com/chemistry/colorimeter/
- https://laboratorytests.org/colorimeter/
- https://collegedunia.com/exams/colorimeter-chemistry-articleid-681
- https://microbiologynote.com/colorimeter-working-principle-definition-parts-uses/
- https://www.testronixinstruments.com/blog/working-principle-applications-of-colorimeters/
- https://microbiologynote.com/colorimeter-working-principle-definition-parts-uses/

