Chelating agents control metal ions by inhibiting the metal ion reactive sites and preventing them from entering their regular (often unwanted) reactions. Chelating substances that establish the most stable bonds with metal ions will be the most effective at preventing metal ion activity.
Chelating agents are chemical compounds with at least two functional groups that can engage with a metal ion to produce a ring structure known as a chelate ring. Metal chelates are chemical compounds formed by the interaction of a chelating agent with a metal ion.
These compounds change the chemical composition of the metal, increasing its overall stability and capacity to attach to other substances. The chelating agent is utilized in a variety of applications in the upstream oil and gas industry, including acidizing, scale removal, filter cake removal, wettability alteration, enhanced oil recovery (EOR), and hydraulic fracturing treatments. They are also employed in cleaning, water treatment, medical uses, protein purification, electrophoresis, DNA extraction, and a wide range of other processes.
Chelating agents are chemical compounds that have structures that allow two or more donor atoms (or sites) to bind to the same metal ion at the same time, resulting in the formation of one or more rings. These compounds are also referred to as chelating groups, and the process by which they create rings is referred to as “chelation.” A chelating agent can generate stable complexes known as chelates through interaction with metal ions at numerous points.
How Chelating Agents Work?
Chelating agents “catch” metals via their ligand-binding atoms. These ligand-binding atoms are electron-sharing atoms that create an interaction with metal ions. As a result, ligand-binding atoms are often referred to as binding electron-donor atoms or binding atoms. Binding atoms are a structural component of a chelating agent and include nitrogen, sulfur, and oxygen.
Electron sharing is a fundamental part of how chelating agents bind to metal ions, and electrons can be shared in two ways: covalent bonds and coordinate linkages. A covalent bond is formed when there is mutual electron sharing, which means that both the chelating agent and the metal ion contribute one electron each to create the bond.
Only the binding atoms of the chelating agent provide the two electrons to bind to the metal ion in the case of a coordinate linkage. Chelation is a term that is often used in various departments of study, including medical sciences, biology and chemical science, science, and clinical sciences. The chelation process is crucial in the detoxification of toxins and the production of complexes.
Types of Chelating Agents
Chelating drugs are usually categorized according to their denticity (or multiplicity)—the number of coordinating groups they include. The simplest chelating agents are called bidentate, those with three coordinating groups are called tridentate, and those with multiple coordinating groups are called multidentate. (The simple coordinating agents are sometimes known as unidentate).
Coordinating groups typically comprise an oxygen, nitrogen, sulfur, or phosphorus atom as the donor atom, with the metal ion acting as the acceptor atom. The bidentate ligands are a particular (and oldest studied) class of chelating agents. EDTA is a chelating agent with a greater number of coordinating groups.
Nomenclature
Chelating agents have formal system names as organic chemicals, but for those that have been in general commercial usage or that chemists utilize as analytical reagents or in extensive investigations of families of compounds, abbreviated or popular names’ (sometimes acronyms) are in common use. Those having commercial applications may have additional trade names, whereas those with pharmacological applications may have ‘drug house’ names. Metal chelates are usually referred to as such, for example, calcium chelate of EDTA, which would be fine if there weren’t so many different alternate names for the same compound, for example, calcium Versenate.
Chelating compounds that are poly acids, polybases, or can operate as both acids and bases are occasionally accessible in a variety of salt forms. All of which can be considered the parent compound. Consider N, N’-1,2-ethanediylbis-[N-carboxymethyl] glycine, an amino carboxylic acid chelating agent with four acidic protons known to most of us as EDTA (the acronym for another scientific term, ethylenediaminetetraacetic acid). It is available commercially as the parent acid, mono-, di-, tri-, and tetrasodium salts, as well as the di-metal salt of various metal chelates, such as the disodium salt of EDTA’s calcium chelate.
Characteristic Properties of Chelating Agent
The following are some characteristics of an ideal chelating agent:
i. They are extremely water soluble.
ii. They can generate nontoxic complexes with hazardous metals.
iii. They are not physiologically active.
iv. They have a higher affinity for metals than the endogenous ligand.
v. They will be readily removed.
Chelation
Chelation, derived from the Greek word indicating claw, is the insertion of a mineral ion or cation into a complex ring structure by an organic molecule, the chelating agent. Sulfur, nitrogen, and or oxygen are common electron-donor atoms on chelating molecules.
The strength of the chemical bonds produced within coordination complexes formed between chelators and metal ions is determined by the elements involved and the stereochemistry.
A range of metal ions that could compete for binding with the chelator (e.g., calcium, magnesium, zinc, copper, manganese, and other metals that typically exceed hazardous element concentrations).
In the medical field identity of the metal primarily bound by a chelating agent is determined by the chelator’s accessibility to the tissues, the strength with which the metal is already bound in the tissues, the strength with which the metal binds to the chelator, and, to some extent, the relative quantities of various ions.
Factors affecting chelation
Effects of other metal ions
Despite the minor effects of ionic strength, only metals that form stable chelates will influence the chelation reaction. Their impacts will be fairly predictable based on the stability constants. In general, given metals with stability constants that differ by three log units, the metal that forms the most stable chelate will be chelated first.
Effects of other anions
Other anions normally have little influence on the chelation process. That is why chelating compounds such as EDTA, HEDTA, and DTPA are so commonly utilized as metal-control agents. The metal chelates are sufficiently stable that the average anion in the system is unable to displace and react with the chelated metal.
Influence of ionic strength
Because chelating agent-metal ion systems are in ionic equilibrium, the total concentration of normally inert ions influences chelating efficiency. As a result, excessive quantities of sulfates, chlorides, phosphates, sodium ions, potassium ions, and other non-chelating molecules tend to lower the metal chelate’s stability constant.
Influence of PH
As chelation is an equilibrium process, a substance that can interact with any of the species existing in the system changes the entire system. Since both the metal ion and the chelating agent are pH sensitive, hydrogen ions disrupt the equilibrium. Since EDTA, DTPA, and HEDTA are polycarboxylic acids, the pH dictates specific ionic species are present in the system.
Chelates
Metal chelates are chemical compounds formed by the interaction of a chelating agent with a metal ion. Metal chelates are a subclass of metal coordination compounds, which is a very wide class. These occur when coordinating agents, chemical substances containing a functional group that can interact with a metal ion, interacts in such a way that they surround it. The term ‘ligand’ is frequently used in place of a coordinating agent (and also to refer to the functional group’s active site or atom). Chelates are chemical compounds that consist of a metal ion and a chelating agent. A chelating agent is a chemical whose molecules have the ability to create many bonds with a single metal ion. In other words, a chelating agent is a multidentate ligand.
Example of formation of chelate
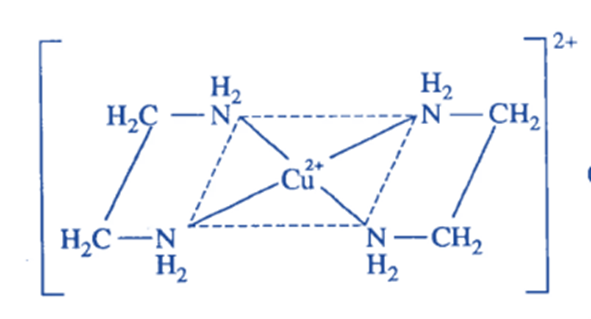
Cu(en)2 2+ complex is generated when two molecules of ethylene diamine (en), a bidentate ligand, are connected with one cu+ ion via its two N-donor atoms of each molecule. The chelating ligand used in the formation of this complex is ethylene diamine, and the resulting complex ion is known as chelates.
Factors affecting the stability of chelates
The following are major elements that influence chelate stability.
1. The chelate ring’s size. Chelates with 6-membered rings that include the metal atom are more stable than those with 5-membered rings, which are more stable than chelates with rings, and so on.
2. The total number of chelate rings. Greater The greater the number of chelate rings, the more stable the chelate.
3. The effects of resonance. The chelate’s stability is improved by resonance.
4. The chelating action. Chelated complexes are known to be more stable than non-chelated complexes.
This is known as the chelate effect.
Chelating effect:
The chelating effect is the increased affinity of chelating ligands for a metal ion relative to non-chelating (monodentate) ligands for the same metal. The stronger the chelating impact, the more stable the resultant complex. [cu(en)] has a greater stability constant than [cu(Me NH2)].
Chelates are thought to be more stable than non-chelated substances. The greater the extent of chelation, the more stable the combination will be due to the increased number of ring linkages to the metal atom. This is known as the chelate effect.
The number of atoms present in the chelate ring directly indicates how stable the chelate is. Chelates having a formation of six or five-membered rings are often found to be more stable than those with eight, seven, and four-membered rings. The chelating ligand significantly increases the stability and strength of complexes. This is known as the chelate effect.
Examples of chelates
• Metal cations can be broken down by the majority of biological particles, resulting in the creation of chelate complexes. Polynucleic acids, polypeptides, proteins, polysaccharides, and amino acids are examples of polydentate ligands.
• A bidentate ligand, ethylenediamine, forms a five-membered ring with the chelate complex and copper ion.
• Chelated metals, which are frequently cofactors, prosthetic groups, and peptides, are found in the majority of metalloenzymes.
• Natural metal ion chelates extracted from rocks and minerals are frequently responsible for hot chemical weathering.
• Chelated structures are commonly homogeneous catalysts, such as ruthenium (II) chloride chelation with bidentate phosphine.
Applications of Chelating Agents
In Agricultural
Metal chelate compounds are common fertilizer components that deliver micronutrients. Micronutrients (Mn, Fe, Zn, Cu) are necessary for plant growth. Most fertilizers contain phosphate salts, which in the absence of chelating agents often transform these metal ions into insoluble solids with no nutritional value to the plants.EDTA is a common type of chelating agent that keeps these metal ions soluble. Some chelating chemicals can be applied directly to the soil for root uptake, while others can be applied as foliar sprays. Some are also well suited for usage in soilless culture since precipitates do not develop within the active pH ranges. EDTA and DTPA are the most commonly utilized chelates in agriculture.
Dental applications
The dentin adhesives method was based on a co-monomer chelating ligand with calcium on the surface of the tooth.
EDTA is often used in dentistry to clean and shape canals. Since EDTA chelates with metallic ions in the medium, which are required for microbe growth, it slows bacterial growth and eventually kills them via starvation.
Pharmaceutical applications
Gadolinium chelate complexes are frequently employed as contrast agents in MRI examinations.
Arsenic poisoning is treated with BAL. It has two unsaturated sulphydryls (-SH) groups that react with metal. BAL’s two -SH groups combine with arsenic and prevent it from combining with the -SH groups of the respiratory enzyme system. In copper poisoning and Wilson’s illness, as an adjuvant to penicillamine.
Penicillamine has a high copper chelating capacity and was used to treat Wilson’s illness in 1956. It specifically chelates Cu, Hg, Pb, and Zn.
In catalysis
Bidentate phosphine in noyori asymmetric hydrogenation and manufacture of synthetic (-)-menthol.
in oils production
Chelating compounds are utilized in the oil industry for stimulation treatment and cleaning surface facilities. EDTA, HEDTA, NTA (nitrilotriacetic acid), and citric acid are examples of common oilfield chelating agents.
Geology
Chelating agents can be employed in geology to study chemical weathering.
Separation of ions by solvent extraction method
Dimethyl glyoxime, acetylacetone, trifluoroacetyl acetone, 8-hydroxyquinoline (oxine), dithioxone, cupferron, and other chelating ligands form chelates with numerous metallic ions.
These chelates have no charge, making them neutral complex molecules known as inner complexes. These compounds are typically insoluble in water but can be dissolved in organic solvents under certain experimental conditions. It is therefore possible to segregate a given ion from other ions by extracting the inner complexes in organic solvents. This process of separating a certain ion from other ions is known as solvent extraction.
In titrimetric analysis
Some chelating agents are used in the titrimetric analysis of metal ions present. For example, EDTA is used in complexometric titration.
Chelation therapy
Chelation treatment is a medical practice that involves the introduction of a chelating substance in order to eliminate heavy metals from the body. In chelation therapy, a variety of chelating compounds are employed as medications to treat heavy metal intoxication.
In addition to the following requirements, the toxicokinetics and toxicodynamics of metals and chelating compounds are essential components of efficient chelation therapy:
i. A strong affinity for the toxic metal.
ii. Low affinity for important metals
iii. Low toxicity
iv. Lipid solubility
v. Excellent absorption from the gastrointestinal system.
Natural chelation
Metal cations can be dissolved by almost any biological process. As a result, proteins and polysaccharides are effective polydentate ligands for a wide range of metal ions. In addition to these random chelating agents, some are designed to bind certain metals. The porphyrin ring of hemoglobin or chlorophyll, as well as the Fe3 + chelated siderophore released by microbes, are examples of chelating agents. Plants commonly use histidine, malic acid, and phytokeratin as chelating agents to avoid free harmful metal ions. Many microbial species create water-soluble pigments that glow when exposed to ultraviolet light. These pigments function as a chelating agent known as a siderophore.
Some Chelating Agents
Ethylenediamine
A single ethylenediamine molecule can make two bonds with a transition-metal ion such as nickel(II), Ni2+. The metal ion forms bonds with the nitrogen atoms of ethylenediamine. Because the nickel(II) ion may create six such bonds, three ethylenediamine molecules can be connected to one Ni2+ ion.
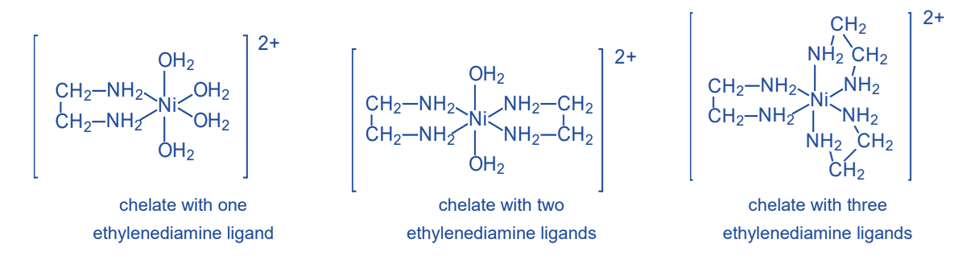
Water molecules complete the bonding capacity of the Ni2+ ion in the two structures on the left. Water is not a chelating agent since each water molecule forms only one bond with Ni2+. Chelates are typically more stable than complexes generated with monodentate ligands, such as water, since the chelating agent is linked to the metal ion by several bonds.
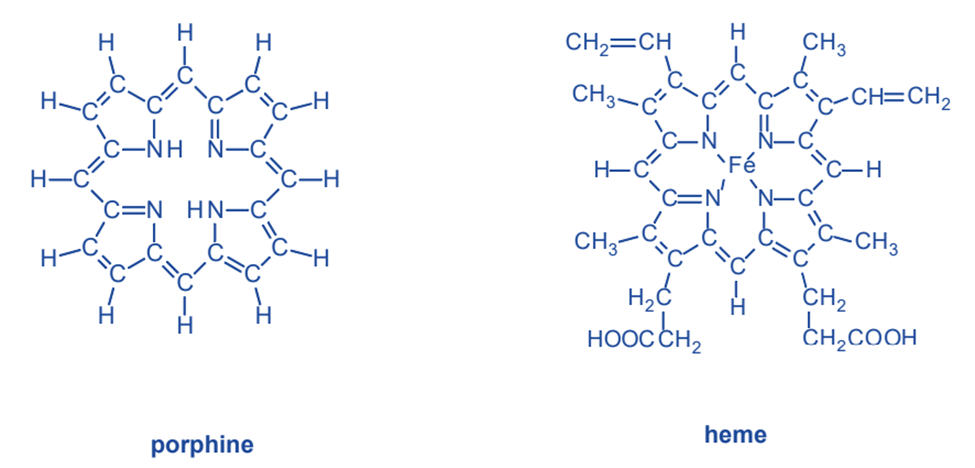
Prophine
Porphine, like ethylenediamine, is a chelating chemical that establishes connections with metal ions via nitrogen atoms. Each of the four nitrogen atoms in the molecule’s core can form a connection with a metal ion. Porphine is the most basic of a class of chelating compounds known as porphyrins. Porphyrins have a structure obtained from porphine by substituting different groups of atoms for part of the hydrogen atoms around the outside. Heme, the major component of hemoglobin, which transports oxygen from the lungs to the tissues, is a significant porphyrin chelate. A porphyrin chelating agent is linked to an iron(II) ion in heme. Iron, like nickel, has the ability to make six bonds. Four of these links connect it to porphyrin. One of iron’s two remaining connections binds an oxygen molecule as it travels through the blood.
Dimercaptol
B.A.L. (British Anti-Lewisite) is another name for dimercaprol or dimercaptopropanol. It functions as a physiological antidote. It has two unsaturated sulphydryl (-SH) groups that react with metal. For example, two -SH groups of BAL bind with arsenic and prevent it from combining with -SH groups of the respiratory enzyme system. Because the complex produced is generally stable and water soluble, it is transported into tissue fluids, notably plasma, and eliminated in urine rather than diffusing into tissue.
EDTA
The quantity of metal in the metal ion solution is calculated using EDTA. An appropriate indicator, such as Erichrome black T, is used during titration. First, when the pH of the solution is set, the indicator forms a complex with metal ions and changes color. To keep the pH stable, a buffer solution is utilized. When EDTA is added to the solution, it forms a complex with the metal ion, freeing the indicator and allowing it to return to its original color.
Metal + In (indicator) → Metal -In complex (red)
Metal -In complex + EDTA → Metal- EDTA + In (blue)
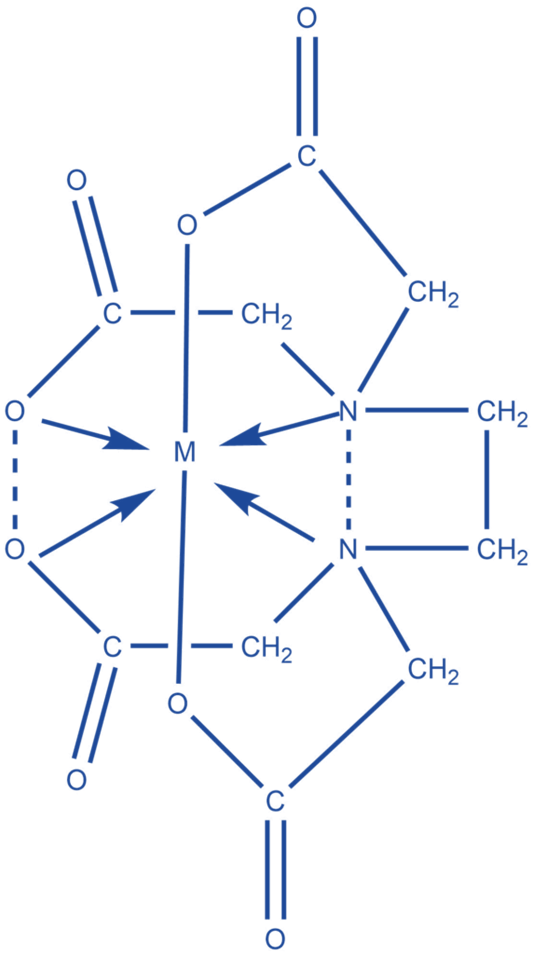
EDTA (Ethylenediaminetetraacetic acid) is a chelating agent that controls metal concentrations in order to modulate enzyme activity. To reduce metal toxicity, which would otherwise destroy DNA molecules, EDTA is frequently used in DNA preservation.
8-Hydroxyquinoline
8-Hydroxyquinoline (8HQ) is a tiny organic molecule with chelating capabilities that are employed in molecular biology as well as medicine, agriculture, biochemistry, and the textile industry.
8HQ has the ability to form complexes with divalent metal ions (metals with two valence electrons), such as calcium, oxygen, zinc, copper, iron, manganese, nickel, and magnesium.
As cofactors in numerous enzymes, these metals serve vital roles in the metabolic equilibrium of life. 8HQ inhibits RNA polymerase activity by chelating ions like magnesium and manganese. It is also used as a chelator in cancer research studies where 8HQ and iron form a complex intercalating with DNA. This causes strong DNA damage inside cancer cells, leading to cell death. Additionally, it is used as a chelator in neurodegenerative disorder studies (Alzheimer’s and Parkinson’s diseases). 8HQ binds to Copper and Zinc ions to inhibit their linkage with amyloid-β (Aβ) peptides implicated in Alzheimer’s disease (AD).
References
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)/22%3A_Metals/22.10%3A_Chelating_Agents.
- https://www.corrosionpedia.com/definition/254/chelating-agent
- https://www.slideshare.net/drdhriti/chelating-agent
- https://www.britannica.com/science/chelating-agent.
- https://pharmafactz.com/medicinal-chemistry-chelation-therapy/
