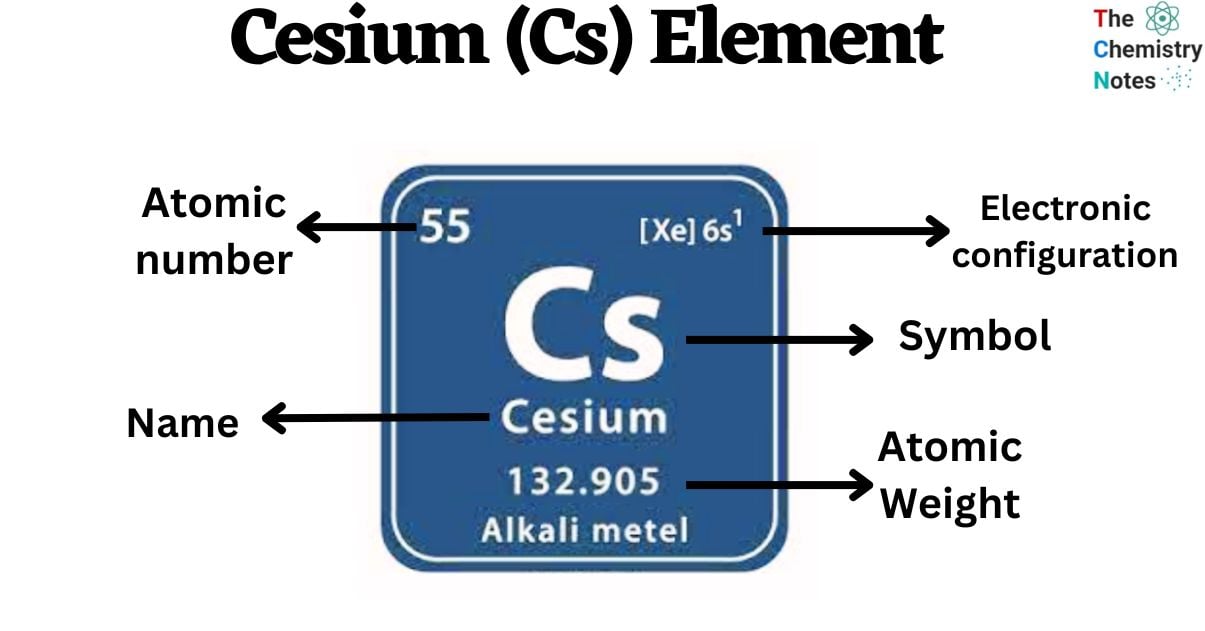
Cesium is a chemical element with the atomic number 55 and is represented by the symbol ‘Cs’ in the periodic table. It is a soft, silvery-golden alkali metal and belongs to the s-block of group 1 of the periodic table. This material has a low melting point of 28 degrees Celsius (83 degrees Fahrenheit), which makes it one of the metals that are liquid at or close to room temperature. Other metals that fall into this category are rubidium (39°C), francium (27 °C), mercury (-39 °C), and gallium (30 °C).
The presence of cesium on the Earth’s crust is estimated to be 3ppm (parts per million) which makes it the 36th most abundant metal and 45th most abundant element overall. It is a relatively rare metal because of its low occurrence. Despite this, it is more common than elements like antimony, cadmium, tin, and tungsten, and it is noticeably more common than mercury and silver. The disparity in the number of occurrences is about equivalent to two orders of magnitude.
Interesting Science Videos
History of Cesium
- Robert Bunsen and Gustav Kirchhoff discovered that cesium could be found in the mineral water that came from Durkheim, Germany, in the year 1860.
- Caesium was the first element to be discovered using a spectroscope, which Bunsen and Kirchhoff had only developed a year before their discovery of the element.
- It was first isolated by the German chemist Carl Setterberg in 1882.
- After noticing the blue lines in its spectrum, they gave the element the name cesium.
- The term comes from the Latin word “caesius“, meaning “sky blue” and that is the color of the cesium line in the spectroscope.
Occurrence of Cesium
- The presence of cesium on the Earth’s crust is estimated to be 3ppm (parts per million) which makes it the 36th most abundant metal and 45th most abundant element overall. It is a relatively rare metal because of its low occurrence.
- Cesium is one of the “incompatible elements” due to its enormous ionic radius. Cesium is most abundant in the liquid phase of magma and forms crystals later. Therefore, zone pegmatite ore bodies generated by this enrichment process are the world’s greatest reserves of cesium.
- Since cesium doesn’t replace potassium as quickly as rubidium does, the alkali evaporite minerals sylvite (KCl) and carnallite (KMgCl3.6H2O) may only have 0.002% of it. As a result, very few minerals contain cesium. Beryl (Be) can contain traces of the element cesium.
- Cesium’s only significant ore is pollucite Cs(AlSi2O6), which is only found in a handful of locations worldwide in zoned pegmatites alongside the more commercially valuable lithium minerals, lepidolite, and petalite.
- The most important and significant source of cesium in the world resides in the Tanco Mine at Bernic Lake in Manitoba, Canada. The Bikita pegmatite vein in Zimbabwe is worked for petalite, but it also has a lot of pollucites. A lot of pollucites can also be found in Namibia’s Karibib Desert.
Isotopes of Cesium
Cesium has only one naturally occurring stable isotope: 133Cs.
Naturally Occurring Isotope of Cesium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 133Cs | 100 |
Elemental Properties of Cesium
| Electronic Configuration | [Xe] 6s1 |
| Atomic Number | 55 |
| Atomic Weight | 132.90 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 1, 6, s-block |
| Density | 1.93 g.cm -3 at 20 °C |
| Ionic radius | 0.167 nm |
| Van der Waals radius | 0.267 nm |
| Electron shells | 2, 8, 18, 18, 8, 1 |
| Electrons | 55 |
| Protons | 55 |
| Neutrons in most abundant isotope | 78 |
Physical Properties of Cesium

- Cesium is a soft metal with a silvery-golden luster.
- It has a boiling point of 671 °C (1240 °F) and a melting point of 28.5 °C (83.3 °F), which makes it liquid at room temperature.
- Cesium is one of the least dense metals. The density of cesium at room temperature is 1.93 g/cm3 and its liquid form density is 1.843 g/cm3.
- Cesium is ductile when in solid form which means it can be drawn into thin wires without breaking it.
- It is also malleable meaning it can be beaten into thin sheets without any cleavage.
- The electrical conductivity of cesium is higher than that of any other metal, with the exception of silver and copper, both of which have a higher conductivity than cesium.
- Because of the comparatively low effective nuclear charge experienced by its valence electron, cesium has the biggest atomic radius of any of the elements in the periodic table.
- Cesium is sonorous meaning it can produce a ringing sound when struck.
| Color/physical appearance | Lustrous, metallic, silvery-gold |
| Melting point/freezing point | 301.7 K (28.5 °C, 83.3 °F) |
| Boiling point | 944 K (671 °C, 1240 °F) |
| Density | 1.93 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 0.79 (Pauling Scale) |
Chemical Properties of Cesium
- Among all the alkali metals, cesium has the highest rate of chemical reactivity. It produces hydrogen gas and a solution of cesium hydroxide when they come into contact with water in an explosion. Additionally, it has a violent reaction with a variety of other compounds, such as acids and halogens.
- It is pyrophoric in nature which means it can readily ignite in air so it can be handled under an inert atmosphere only.
- There is just one stable state of oxidation for cesium, and that state is +1. When it interacts with other elements, cesium gives up one of its electrons, resulting in the formation of a positively charged ion.
- Because cesium is one of the elements with the highest electropositive potential, it rapidly gives up its valence electron when it participates in chemical reactions.
- Cesium is capable of forming a wide range of different chemical compounds, some of which are halides, carbonates, nitrates, and sulfates. Cesium chloride, cesium carbonate, and cesium nitrate are examples of some of the most significant compounds that include cesium.
Chemical Reaction of Cesium
- The Reaction of Cesium With An Air
The bright and sparkling appearance of cesium’s surface is due to the element’s brilliant softness and ease of cutting. However, in a short amount of time, the surface will start to get tarnished as the outcome of chemical reactions involving oxygen and humidity in the air. When cesium is burnt in the air, the primary byproduct that is produced is an orange compound known as cesium superoxide or CsO2.
Cs (s) + O2 (g) → CsO2 (s) [orange]
- The Reaction of Cesium With Water
Cesium metal quickly interacts with water, forming a colorless solution of cesium hydroxide (CsOH) and hydrogen gas (H2). The dissolved hydroxide causes the resulting solution to become basic. The process is extremely exothermic in nature The reaction is so quick that if it is performed in a glass vessel, it will shatter.
2 Cs (s) + 2 H2O (l) → 2 CsOH (aq) + H2 (g)
- The Reaction of Cesium With Halogens
Cesium reacts vigorously with all the halogens to form cesium halides.
Cesium reacts strongly with fluorine, F2, to produce cesium (I) fluoride, CsF.
2 Cs (s) + F2 (g) → CsF (s)
Cesium reacts strongly with chloride, Cl2, to produce cesium (I) chloride, CsCl.
2 Cs (s) + Cl2 (g) → CsCl (s)
Cesium reacts strongly with bromide, Br2, to produce cesium (I) bromide, CsBr.
2 Cs (s) + Br2 (g) → CsBr (s)
Cesium reacts strongly with iodine, I2, to produce cesium (I) iodide, CsI.
2 Cs (s) + I2 (g) → CsI (s)
- The Reaction of Cesium With An Acid
Cesium metal is easily dissolved in dilute sulfuric acid, which results in the formation of solutions that contain the aquated Cs(I) ion in addition to hydrogen gas, H2.
2 Cs (s) + H2SO4 (aq) → 2 Cs+ (aq) + SO42- (aq) + H2 (g)
Uses of Cesium
In the fields of science, technology, and medicine, cesium can be used for a wide number of objectives, a few of which are going to be covered below.
- Used In Petro-chemical Industries
Formate brines are a high-density, low-viscosity fluid used for high-pressure and high-temperature oil and gas drilling exploration. Cesium is utilized in the production of these brines.
Cesium formate is used to maintain well control and protect drilling polymers due to its antioxidant activity. Cesium-based aqueous solutions are frequently utilized in the oil industry as drilling and completion fluids.
- Used In Atomic Clocks
The electromagnetic transitions in the hyperfine structure of the caesium-133 atoms serve as a reference for Cs-based atomic clocks. Cs are additionally employed as highly accurate oscillators in fiber-optic telecommunication synchronization. Cs clocks use the microwave radiation cycles that the electrodes of cesium produce as a measure of time. The worldwide definition of a second is based on the Cs atom due to the exceptional accuracy of the atomic clock.
- Used In Electronics
Using cesium as a photoemitter, free electrons are created from photons. In photoelectric cells, cesium is used for the intermetallic cathodes. Scintillation counters, which transform ionizing radiation’s power into pulses of visible light, employ Cs. To prepare lenses, prisms, and cuvettes that are for utilization in infrared spectrometers, particularly in the 500–550 nm region, cesium is utilized. High-powered lasers, vapor rectifiers, and vapor glow lamps have all employed Cs.
- Used In Mechanical Industries
When cesium is added it can change the index of refraction of silicate or borosilicate glass. It is also used to make fluxes for brazing magnesium-containing aluminum alloys. Using cesium compound melts or solutions, surface ion exchange can make glass surfaces resistant to rust or breaking. Laser light’s frequency can be changed using Cs-Li-borate crystals. It could be used to fix radioactive waste in cesium-based glass and in fugitive emission cure processes. It has been added as carbonates to glass to make fiber optics and night vision devices more stable and long-lasting.
Health Effects of Cesium
- The quantity of cesium in food and drinks is determined by the release of radioactive cesium from nuclear power plants, which occurs primarily as a result of accidents.
- Cesium can enter a person’s body when they breathe, drink, or eat. Cesium amounts generally remain low in the air, but radioactive cesium has been found in surface water and in several types of food.
- Cesium-related health consequences are unlikely. Exposure to radioactive cesium is capable of harming cells in rare cases. Nausea, vomiting, diarrhea, and bleeding may result. Long-term exposure can cause unconsciousness. Coma or death may ensue. Individual resistance, duration, and concentration determine the severity of the effects.
Environmental Effects of Cesium
- Cesium is naturally found in rocks and minerals. It’s released into the air, water, and land during ore mining and milling. Nuclear power plants, accidents, and nuclear weapons tests may discharge radioactive particles into the air.
- Cesium can travel far before landing on Earth. Cesium compounds are highly soluble in water and soil. Cesium is not transported away from soils into groundwater. Since it firmly bonds to soil particles, plant roots cannot absorb it. Radioactive cesium can penetrate plants via leaves.
References
- https://www.britannica.com/science/cesium
- https://www.rsc.org/periodic-table/element/55/caesium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011.
- https://pubchem.ncbi.nlm.nih.gov/element/Cesium
- https://www.chemicool.com/elements/cesium.html
- https://www.livescience.com/37578-cesium.html
- https://byjus.com/chemistry/cesium/
