Cement is a substance of inorganic nature that encompasses a composite blend of calcareous and argillaceous components. Cement, a paramount construction material, functions as a cohesive agent that solidifies and fortifies its adherence to architectural components, including stones, bricks, tiles, and the like. The term “cement” typically denotes a finely ground particulate material primarily composed of limestone (calcium), sand or clay (silicon), bauxite (aluminum), and iron ore. It may also incorporate additional constituents such as shells, chalk, marl, shale, clay, blast furnace slag, and slate.

Cement plays a pivotal role in the development and maintenance of urban infrastructure. The substance in question finds application in the production of concrete and mortar, serving the purpose of fortifying the structural integrity of infrastructure through the process of binding individual building components together.
The composition of concrete typically comprises specific proportions of cement, water, sand, and gravel, while the mortar is primarily composed of cement, water, and lime aggregate. Both of these materials are employed in the process of securing rocks, stones, bricks, and other building units, as well as filling or sealing any voids, while also serving the purpose of creating aesthetically pleasing decorative patterns. The amalgamation of cement, water, silicates, and aluminates engenders a hydrophobic, solidified aggregate that finds application in the realm of water resistance.
Interesting Science Videos
Origin and History of Cement
- The ancient civilizations of Babylonians and Assyrians utilized bitumen as a binding agent for burnt brick or alabaster slabs. In Ancient Egypt, the construction practice involved the use of a mortar composed of sand and gypsum (CaSO4 · 2H2O), which was typically partially calcined and occasionally contained calcium carbonate (CaCO3).
- Calcium oxide, commonly known as lime, was utilized in the historical context of Crete and by the Ancient Greeks. There exists evidence indicating that the Minoans residing in Crete employed crushed potsherds as an artificial pozzolan in the production of hydraulic cement.
- The ancient Greeks utilized volcanic tuff sourced from the island of Thera as their pozzolan material, while the Romans employed crushed volcanic ash, which consisted of activated aluminum silicates, in combination with lime.
- The technological expertise needed to produce hydraulic cement was codified in the 18th century by French and British engineers.
- During the planning phase of the third Eddystone Lighthouse (1759-1857) in the English Channel, John Smeaton made a significant contribution to the advancement of cement technology. The individual required a hydraulic mortar that could undergo a process of solidification and increased strength within a twelve-hour timeframe, specifically during the period of high tides.
- As a result of the render it produced having a hue resembling the famed Portland stone, Joseph Aspdin patented a comparable substance in 1824 and named it Portland cement.
- In 1845, Isaac Charles Johnson conducted an experiment wherein he subjected a mixture of clay and chalk to controlled combustion, resulting in the formation of a clinker. This innovative approach aimed to enhance the quality of cement.
- In 1851, the individual established manufacturing facilities with the purpose of producing cement using the aforementioned combination of materials.
Raw Materials for Cement Production
- Calcareous materials, including limestone, chalk, marble, and alkali waste, are examples of substances with a high content of calcium carbonate.
- Argillaceous materials, including clay, shale, slate, iron stone, and blast furnace slag, are commonly used in various applications due to their unique properties. These materials contain significant amounts of aluminum oxide (AlO2O3) and silicon dioxide (SiO2), which contribute to their desirable characteristics.
- Gypsum, chemically known as calcium sulfate dihydrate (CaSO4.2H2O), is commonly used in construction materials to prevent flash setting.
What the Functions of Ingredients
Lime
- Lime is a fundamental constituent of cement. It is advisable to incorporate an appropriate amount of the substance in order to achieve a significant production of calcium silicates, calcium aluminates, and calcium aluminoferrite hydrate.
- These components hold great importance in the formulation of cement.
- The strength of cement is compromised by an excessive or inadequate quantity of lime.
Silica
- Silica plays a vital role in the composition of cement by significantly improving its strength characteristics.
- The excessive occurrence of slicing can hinder the effectiveness of the setting and hardening procedures.
Alumina
- Alumina, also known as aluminum oxide (Al2O3), plays a crucial role in the initial enhancement of strength in various materials. Nevertheless, an excessive amount of alumina has been found to diminish the overall strength.
Gypsum
- Gypsum is frequently employed as a retarder in cement to effectively slow down the rapid setting process.
Magnesia
- Magnesia, also known as magnesium oxide, is a chemical compound denoted by the chemical formula MgO.
- It is advised to limit the concentration of magnesia in cement to a maximum of 2%.
- The excessive inclusion of magnesium in cement has been observed to result in a reduction in its overall strength.
Iron Oxide
- The chemical formula for iron(III) oxide is Fe2O3.
- Iron oxide is responsible for providing color to cement and it functions as a flux.
- At elevated temperatures, the substance actively participates in a chemical reaction involving calcium and aluminum, resulting in the formation of tricalcium alumino-ferrite.
- Tricalcium aluminoferrite is a compound that plays a crucial role in enhancing the hardness and strength of cement.
Sulfur trioxide
- The presence of sulfur trioxide in tiny amounts has been suggested to be beneficial. This chemical increases the durability and strength of cement.
- When there is an excessive amount, the level of soundness is reduced. The idea of soundness in cement relates to its capacity to withstand variations in volume throughout the hydration process.
- When cement hydrates, it is deemed sound if there is just a slight volume change.
Alkalies
- The presence of the substance should not exceed a threshold of 1%.
- An excessive quantity of alkali can result in the efflorescence of cement, which refers to the removal of water molecules from hydrated crystals.
Steps of Manufacturing Cements
Extraction of Raw Materials
- The production of cement involves the utilization of a blend of raw materials, typically comprising calcium, silicon, iron, and aluminum. The essential components necessary for this procedure encompass limestone, clay, and sand.
- Limestone is frequently employed as a calcium source. The composition consists of considerably smaller proportions of sand and clay. Sand and clay are viable sources for the extraction of silicon, iron, and aluminum.
- Furthermore, a diverse range of raw materials is employed in the cement manufacturing process. Several examples of materials include shale, fly ash, mill scale, and bauxite. The procurement of raw materials is sourced externally in response to the limited demand.
- Before the transportation of raw materials to the cement plant, the initial step involves the utilization of a crusher at the quarry to effectively reduce the size of large rocks into smaller fragments. A crusher is employed to reduce the size of large rocks to that of gravel.
Blending Raw Materials (Crushing and Grinding)
The blending of raw materials can be accomplished through two primary methods: the dry process and the wet process.
Dry process
- The dry process involves the crushing of raw materials, namely limestone, and clay, into fine powder. These materials are then carefully mixed in precise proportions.
- The composition of cement is determined based on the specific properties that are desired for the final product. In most cases, limestone consists of approximately 80% calcium carbonate, while the remaining 20% is composed of clay minerals.
- In the cement manufacturing process, the raw mix undergoes a drying process to reduce its moisture content to less than 1%.
- This dried mixture, known as the raw mix, is then prepared for introduction into the rotary kiln.
Composition of Raw-Mix
| Components | Proportion (%) |
|---|---|
| Lime (CaO) | 60-69 |
| Silica (SiO2) | 17-25 |
| Alumina (Al2O3) | 3-8 |
| Iron Oxide (Fe2O3) | 2-4 |
| Magnesium Oxide (MgO) | 1-5 |
| Sulfur trioxide (SO3) | 1-3 |
| Alkali Oxides (Na2O + K2O) | 0.3-1.5 |
Wet Process
- In the wet process of cement production, calcareous materials, primarily composed of calcium oxide (CaO), undergo a series of steps.
- These materials are first crushed and then finely powdered before being stored in large tanks for further processing.
- In the process of preparing argillaceous materials, such as Al2O3 and SiO2, it is common practice to wash them with water in order to eliminate any impurities present.
- Once the washing process is complete, these materials are typically stored for further use or processing.
- The raw materials are carefully blended in appropriate ratios and subsequently transformed into a paste through the utilization of a grinding mill.
- The substance referred to as “slurry” is a type of paste. The slurry is subsequently transferred into the rotary kiln for the purpose of undergoing combustion.
Differences Between Dry and Wet Process
| Dry Process | Wet Process |
|---|---|
| Low production cost | High production cost |
| Slower process | Faster process |
| Lower fuel consumption | Higher fuel consumption |
| Cement is of Inferior quality | Cement is of superior quality |
Pre-heating Raw Materials
- After the final grinding process is completed, the material is ready to be prepared for the pre-heating chamber.
- The pre-heater chamber consists of a series of vertical cyclones through which the raw material passes before entering the kiln.
- The pre-heating chamber has been specifically designed to effectively utilize the high-temperature gases that are produced by the kiln.
- The practice of pre-heating the material provides numerous benefits, such as energy preservation and improved environmental sustainability within the facility.
Kiln Phase
The kiln phase is a crucial stage in the cement production process. The production of clinker involves a sequence of chemical reactions between calcium and silicon dioxide compounds, resulting in the formation of the desired product from the raw mix.
- The choice between utilizing dry powder or wet slurry in the initial stage is dependent on the specific process employed. Subsequently, the selected material is incinerated in a rotatory kiln.
- A rotary kiln is comprised of rotating steel drums or tubes. The interior of the kiln is lined with refractory bricks. The rotary kiln is inclined at a slight downward angle towards the exit end. The apparatus is affixed to rollers and has the capability to rotate at various speeds, such as one revolution per minute (1 rpm), along its longitudinal axis.
- As the kiln undergoes rotation, the charge gradually descends. The fuel-air mixture is introduced at the lower end, resulting in the heating process. The temperature achieved within the kiln ranges from 1300 °C to 1500 °C.
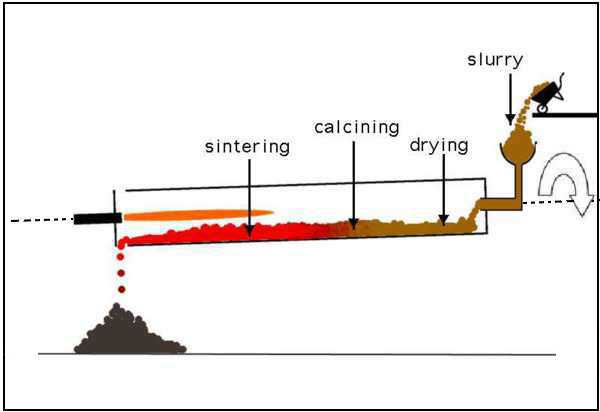
The kiln is comprised of the following zones:
Drying Zone
- The uppermost section of the kiln is commonly known as the drying zone, where a consistent temperature of 400 °C is maintained.
- At the specified temperature, the water within the slurry undergoes the process of evaporation.
Calcinated Zone
- The area within the kiln commonly known as the calcination zone is meticulously controlled in order to sustain a temperature of roughly 1000 °C.
- The powdered limestone present in the raw mix or slurry undergoes a process of breakdown, leading to the generation of quicklime, which is also referred to as calcium oxide (CaO).
CaCO3 (s) → CaO (s) + CO2 ↑ (g)Clinker Zone
- The lower portion of the kiln, known as the clinkering zone, is a significant section of the kiln’s operation.
- The temperature is consistently regulated within the range of 1300 to 1500 °C. When quicklime and clay are mixed together, they undergo a chemical reaction that leads to a fusion process.
- The resulting compounds are calcium silicates, calcium aluminate, and calcium aluminoferrite. Abbreviations are frequently employed in industrial settings to denote chemical raw materials.
2 CaO + SiO2 → 2CaO.SiO2 or 2C + S → C2S3 CaO + SiO2 → 3CaO.SiO2 or 3C + S → C3S3 CaO + Al2O3 → 3CaO.Al2O3 or 3C + A → C3A4 CaO + Al2O3 + Fe2O3 → 4CaO.Al2O3.Fe2O3 or 4C + A + F → C4AF- The process of compound fusion takes place as a consequence of the high temperature experienced during clinkering, leading to the creation of a liquid phase.
- Throughout the descent phase, the liquid substance experiences a series of changes, notably solidification, and cooling.
- These transformations lead to the creation of clinker, which can be described as stones exhibiting a grayish coloration.
Final Grinding
- Once the clinker has undergone the cooling process, it is discharged and subsequently transported to the tube mills.
- Within these mills, the clinker undergoes a process of meticulous grinding in order to attain the required degree of fineness. This level of fineness is determined by the specific classification of the desired end product.
- The process of finely grinding clinker leads to a phenomenon known as rapid setting, which occurs as a result of the material’s high capacity to absorb moisture from the surrounding atmosphere.
- To effectively control the process, a retarding agent called gypsum (CaSO4.2H2O) is commonly employed. This substance is added in a proportion of approximately 2% of the total weight.
- The term “cement” refers to the composite material formed by the combination of clinker and gypsum. After the completion of the manufacturing process, the final product, referred to as Portland cement, proceeds to the packaging stage.
Chemical Reaction During Cement Production
Clay Decomposition
Si2Al2O5(OH)2 → 2 SiO2 + Al2O3 + 2 H2O ↑KAlSi3O8 + 0.5 SO2 + 0.25 O2 → 3 SiO2 + 0.5 Al2O3 + 0.5 K2SO4Dolomite Decomposition
CaMg(CO3)2 → CaCO3 + MgO + CO2KMg3AlSi3O10(OH)2 + SO2 + O2 → K2SO4 + 3 MgO + 0.5 Al2O3 + 3 SiO2 + H2O ↑Low-Temperature Calcite Decomposition
2 CaCO3 + SiO2 → Ca2SiO4 + 2 CO22 MgO + SiO2 → Mg2SiO4Ca5(PO4)3OH + 0.25 SiO2 → 1.5 Ca3(PO4)2 + 0.25 Ca2SiO4 + 0.5 H2O ↑Alumina and Oxide Reactions
12 CaCO3 + 7 Al2O3 → Ca12Al14O33 + 12 CO24 CaCO3 + Al2O3 + Fe2O3 → Ca4Al2Fe2O10 + 4 CO24 CaCO3 + Al2O3 + Mn2O3 → Ca4Al2Mn2O10 + 4 CO2The Reaction of Remaining Calcite
CaCO3 → CaO + CO2Sintering
Ca2SiO4 + CaO → Ca3SiO5Different Types of Cement
Portland cement is a finely ground substance that is produced through the process of calcination. This process involves heating a carefully proportioned mixture of clay-containing materials (known as argillaceous) and lime-containing materials (known as calcareous) to a temperature of 1500 °C. Additionally, a retarder called gypsum is added to the mixture before the calcination process takes place. The resulting product is known as Portland cement. Specialized Portland cement is produced to cater to specific applications. Among the various factors under consideration, the following holds the utmost significance:
Ordinary Portland Cement (OPC)
- This particular variety of cement is commonly known as grey cement.
- It is distinguished by its composition, which consists of 95% clinker and 5% gypsum.
- The type of cement stated here is widely applicable to a range of modern civil engineering projects.
Portland Pozzolana Cement (PPC)
- Portland Pozzolana Cement (PPC) is a type of cement that typically consists of 80% clinker, 15% pozzolana, and 5% gypsum.
- Limestone and other materials are heated to high temperatures to produce clinker, which is the main component of cement.
- Pozzolana is a natural or artificial material that is added to cement to enhance its properties, such as durability.
- The manufacturing process involves the utilization of fly ash, burned clay, coal water, and other relevant materials.
- Volcanic ash is composed of Calcium (Ca), iron, and aluminum silicates.
- When combined with lime and subjected to high temperatures, it undergoes a chemical reaction that results in the formation of pozzolana cement.
Portland Slag Cement (PSC)
- This particular variety of cement is classified as a blended cement, which is produced through two methods: grinding Portland clinker with gypsum and granulated slag or blending ground granulated blast furnace slag with ordinary Portland cement (OPC).
- The composition of Portland Slag Cement (PSC) typically consists of 40% clinker, 55% slag, and 5% gypsum.
White Cement
- Portland cement is typically characterized by its grayish color. The observed color is a result of the formation of complexes with iron oxide.
- When the concentration of iron oxide in cement is decreased to below 0.4%, a noticeable change in color occurs, resulting in the cement appearing white.
- The utilization of this material is primarily limited to decorative purposes due to its relatively high cost.
High Alumina Cement
- The process involves the fusion of a combination of limestone and bauxite at temperatures ranging from 1500 to 1600 °C within a rotary kiln.
- The setting time of this material is comparable to Ordinary Portland Cement (OPC), however, its hardening process is notably accelerated, resulting in the attainment of full strength within a span of 24 hours.
- High-alumina cement exhibits exceptional resistance to elevated temperatures as well as the corrosive effects of seawater and sulfuric acid (H2SO4).
Water Proof Cement
- Waterproof cement is a variant of cement that exhibits certain similarities to Ordinary Portland Cement (OPC).
- Nevertheless, this substance does incorporate a limited quantity of additives, namely calcium stearate, aluminum stearate, or non-saponifiable oil.
- Additives are commonly used in cement to improve its water-proofing properties. This particular substance is widely known in scientific circles as hydrophobic cement.
Quick Setting Cement
- This particular variety of cement is commonly employed in the construction of substantial concrete structures, including dams, piers, and similar projects.
- It is imperative to maintain a reduced level of hydration. In order to minimize the occurrence of construction cracks, certain measures can be implemented.
Rapid Hardening Cement
- The material being discussed shares similarities with Ordinary Portland Cement (OPC), but its main difference lies in its smaller particle size.
- This specific type of cement is commonly referred to as high early-strength cement.
Portland Blast Furnace Slag Cement (PBFSC)
- PBFSC, an acronym for Portland Blast Furnace Slag Cement, is a type of cement that consists of 45% clinker, 50% blast furnace slag, and 5% gypsum.
- This particular type of cement accounts for 10% of the total cement consumption. It has a lower heat of hydration compared to PPC and is commonly used in the construction of dams and other large-scale structures.
Video on Manufacturing of Cement
References
- https://www.britannica.com/technology/cement-building-material
- https://civiltoday.com/civil-engineering-materials/cement/106-cement-manufacturing-process
- https://civiltoday.com/civil-engineering-materials/cement/81-cement-definition-and-full-details
- https://www.sciencedirect.com/topics/engineering/cement-chemistry
- Lavagna L, Nisticò R. An Insight into the Chemistry of Cement—A Review. Applied Sciences. 2023; 13(1):203. https://doi.org/10.3390/app13010203
- https://www.cement.org/cement-concrete/how-cement-is-made

