The cell potential is the measure of potential difference between two half cells in an electrochemical cell. It is represented by the symbol Ecell. In order to create effective and efficient energy sources, engineers need to possess the ability to calculate electrical potentials. An effective electric current can be conducted by obtaining different cell potentials through the comparison and combination of reduction reactions.
Interesting Science Videos
Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy through redox reactions.
Various chemical reactions, such as those occurring in the batteries of your remote or the motor of your car, generate electricity by means of electron transfer. The voltage difference between two half-cells in a battery is determined by the cell potential. In this session, we will discuss the process of determining the voltage in an electrochemical cell and the various components that are involved in it.
Components of Electrochemical Cells
- two half-cells
- two metallic electrodes
- A voltmeter
- salt bridge
An electrochemical cell is composed of two half cells. In an electrochemical cell, one half-cell undergoes oxidation of a metal electrode, while the other half-cell undergoes reduction of metal ions present in the solution. In the half cell, a metal electrode made of a specific metal is immersed in an aqueous solution that contains ions of the same metal. The electrode is linked to the second half-cell, comprising a metal electrode that is immersed in an aqueous solution of metal ions. In this situation, the first half cell will be designated as the anode. In this half cell, the metal atoms present in the electrode undergo oxidation and combine with the other metal ions already present in the aqueous solution.
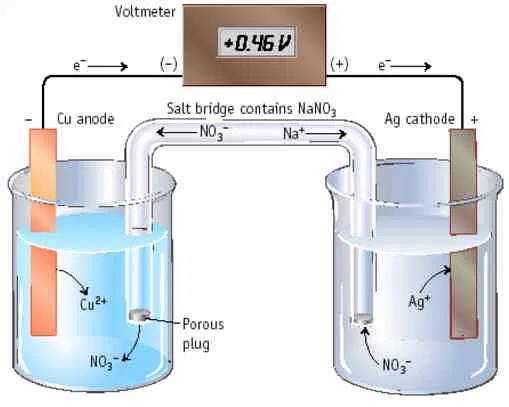
[Image source: Collegeduniya]
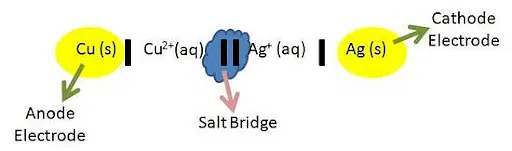
Cell Diagram
The electrochemical cell’s total reaction is depicted in the cell diagram. The substances that are reacting in reduction and oxidation reactions are those that are involved. (Spectator ions are not accounted for.) The cathode half cell is typically written on the right side of the cell diagram, while the anode half cell is always written on the left side. The anode and cathode are separated by two vertical lines.
The anode and cathode solutions’ electrodes are separated by a solitary vertical line. Other compounds that are present in the aqueous solution are added to the graphic after a comma and the chemical. The cell diagram makes it simpler to see what is being reduced and what is being oxidized. These are the chemical processes that create the potential of the cell.
What is Cell potential?
Cell potential refers to the potential difference that is generated at both electrodes. The difference in potential generated at each half cell is generally referred to as cell potential.
E⁰cell = E⁰cathode – E⁰anode
For a spontaneous process, the value of Ecell should be greater than zero.
Cell potential is also called the emf of the cell. It is measured in Volts(V).
EMF = Reduction potential of cathode – Reduction potential of anode
Cell Potential is also related to Gibbs free energy.
The equation for Gibbs free energy change, ∆G, is equal to negative n times the product of the cell potential, Ecell, and Faraday’s constant, F.
∆G = -nEcellF
How to Determine Cell Potential?
- The cell voltage, or the amount of energy generated by the electrodes, is measured by the gold-colored voltmeter at the very top.
- This voltmeter measurement yields the electrochemical cell voltage. Another name for this is Ecell, or the potential difference between the half cells.
- The units of measurement for the energy that each electrical charge contains are called volts; 1 volt equals 1 joule and 1 coulomb.
- The voltage effectively drives the electrons. Electrons are very mobile when there is a high voltage. The voltmeter measures the flow of electrons from the anode to the cathode in Joules per Coulomb.
Standard Cell Potential
The standard cell potential (E0cell) is the difference between the two electrodes that generates the voltage in the cell. The difference between the two half cells is calculated using the equation below:
E0Cell = E0Red, Cathode = E0Red, Anode
E0Cell: is the standard cell potential
E0Red, Cathode: is the typical reduction potential for the reduction half-reaction that takes place there.
E0Red, Anode: is the normal reduction potential for the oxidation half-reaction occurring at the anode.
Calculating Non-Standard State Cell Potentials
When the conditions are different from the standard state (concentrations greater than 1 molar and/or pressures greater than 1 atmosphere),
How do you calculate the cell potential?
- Calculate the cell potential in the standard state.
- Find the new cell potential as a result of the altered circumstances.
- Calculate Q: The reaction quotient
- Calculate n: The number of electrons transferred in the reaction
- Using the Nernst equation, ascertain Ecell, the cell potential in the non-standard state conditions.
Ecell = Eocell – (RT/nF) ln Q
- Ecell = cell potential at non-standard state conditions
- Eocell = standard state cell potential
- R = constant (8.31 J/mole K)
- T = absolute temperature (Kelvin scale)
- F = Faraday’s constant (96,485 C/mole e–)
- n = number of moles of electrons transferred in the balanced equation for the reaction occurring
- in the cell
- Q = reaction quotient.
If the temperature of the cell remains at 25oC, the equation simplifies to:
Ecell = Eocell – (0.0257/n) ln Q
In terms of log10: Ecell = Eocell – (0.0592/n) log Q
Calculation of Cell potential
Estimate the cell potential for the subsequent reaction. when the hydrogen ion concentration is 0.10 M, the bromide ion concentration is 0.25 M, and the oxygen gas pressure is 2.50 atm.
O2(g) + 4 H+(aq) + 4 Br–(aq) 2 H2O(l) + 2 Br2(l)
- Calculate the standard cell potential for the reaction, Eocell, using the tabled values:
| oxidation: | 4 Br–(aq)  ” height=”14″ width=”15″> 2 Br2(l) + 4 e– ” height=”14″ width=”15″> 2 Br2(l) + 4 e– | Eoox. = – Eored. = – (+ 1.077 V) = – 1.077 V |
| reduction: | O2(g) + 4 H+(aq) + 4 e- 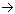 ” height=”14″ width=”15″> 2 H2O(l) ” height=”14″ width=”15″> 2 H2O(l) | Eored. = + 1.229 V |
| overall: | O2(g) + 4 H+(aq) + 4 Br–(aq) | Eocell = + 0.152 V |
- Determine the new cell potential resulting from the changed conditions.
- Calculate the value for the reaction quotient, Q. (Note: We calculate Q using molar concentrations for solutions and pressures for gases. Water and bromine are both liquids, therefore they are not included in the calculation of Q.)
- Calculate the number of moles of electrons transferred in the balanced equation, n.
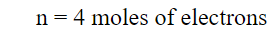
- Substitute values into the Nernst equation and solve for the non-standard cell potential, Ecell.
Ecell = + 0.152 V – (0.0257/4) ln(1.02 x 106)
Ecell = 0.063 V
References
- https://www.chem.purdue.edu/gchelp/howtosolveit/Electrochem/Electrochemical_Cell_Potentials.htm
- https://courses.lumenlearning.com/chemistryformajors/chapter/standard-reduction-potentials/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Voltaic_Cells/The_Cell_Potential
- https://study.com/academy/lesson/electrochemistry-free-energy-and-cell-potential-energy.html
- https://collegedunia.com/exams/cell-potential-definition-diagram-reactions-and-difference-chemistry-articleid-1904#Questions
- https://www.nagwa.com/en/explainers/269168626823/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/cell-potential/
- https://unacademy.com/content/jee/study-material/chemistry/cell-potential/
- https://www.ck12.org/section/cell-potential/
