A catalyst is a substance that initiates or accelerates the rate of a particular chemical reaction without itself being chemically affected. A catalyst can be added to a reaction and then recovered and reused after the reaction occurs. The process or action by which a catalyst increases the reaction rate is called catalysis.
The term catalysis was proposed in 1835 by the Swedish chemist Jöns Berzelius (1779–1848).
Interesting Science Videos
Characteristics of Catalyst
Some properties of catalysts are:
- A catalyst improves the speed of a reaction.
- A catalyst takes part in the reaction even though it will not be consumed or used up in the course of the reaction.
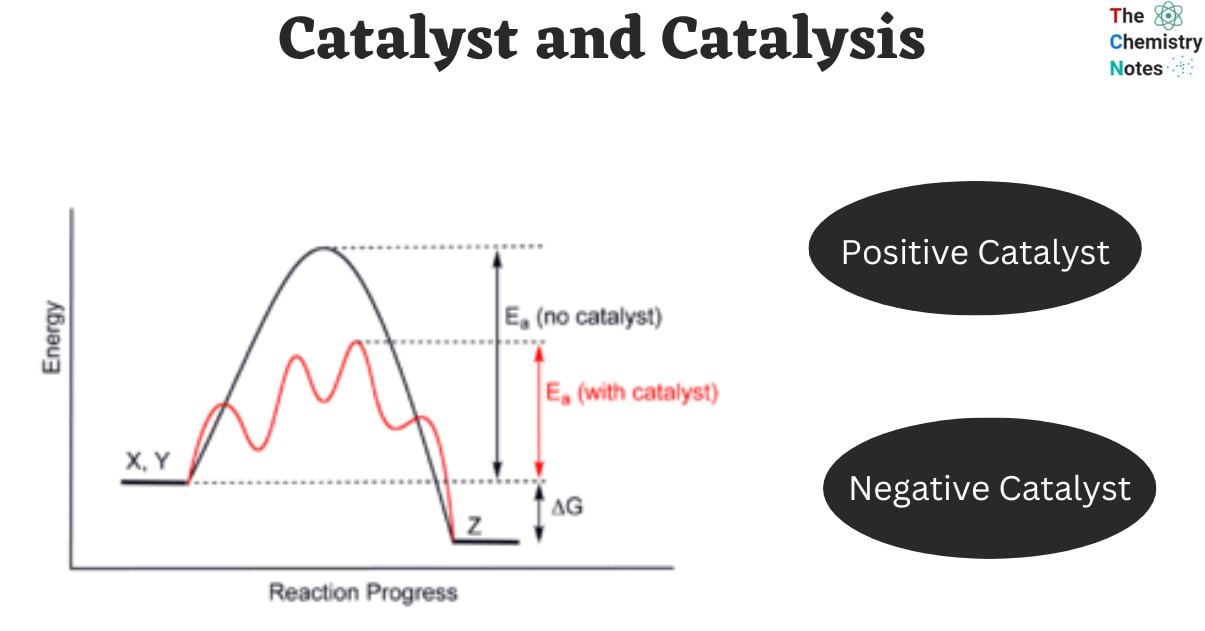
- A catalyst makes the reaction faster by giving an alternative path with lower activation energy.
- In a reversible reaction, a catalyst accelerates the reaction in both directions. Therefore, the inclusion of a catalyst will not change the equilibrium point.
- A catalyst can regenerate after the entire process.
- An extremely small quantity of catalyst causes a considerable increase in the rate of reaction.
- The activation energy of a catalyzed reaction is always lower than that of the same reaction when it is uncatalyzed.
Types of Catalysts with Examples
Depending on the needs or requirements of the chemical process, various types of catalysts may be used.
Positive Catalysts
Positive catalysts are those that increase the rate of a chemical reaction. It accelerates the reaction by lowering the activation energy barriers, allowing a large number of reaction molecules to be converted into products, increasing the percentage of product yield.
Example: Iron oxide acts as a positive catalyst in Haber’s process, increasing the yield of ammonia despite less nitrogen reaction.
Negative Catalysts
Catalysts that slow down the rate of the reaction, as well as negative catalysts It reduces the rate of reaction by increasing the activation energy barrier, which reduces the number of reactant molecules that can be converted into products and thus the rate of reaction.
Example: the decomposition of hydrogen peroxide into water and oxygen is slowed by the use of acetanilide, which acts as a negative catalyst to slow the rate of hydrogen peroxide decomposition.
Difference between a Positive catalyst and a Negative catalyst
There are four main types of catalysts: homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. However, the effect of a catalyst on the rate of reaction can be either positive or negative.
The main distinction between positive and negative catalysts lies in their effects on the reaction rate. Positive catalysts are substances that can enhance the reaction rate, while negative catalysts are substances that can reduce the reaction rate. A positive catalyst functions by reducing the activation energy, thereby accelerating the reaction. On the other hand, a negative catalyst is unable to decrease the activation energy, resulting in a decrease in the reaction rate.
| Positive catalyst | Negative catalyst |
| 1. The substances which decrease the rate of a chemical reaction are called Negative catalysts. | 1. The substances which decrease the rate of a chemical reaction are called Negative Catalyst. |
| 2. It decreases the Activation energy of reactant molecules. | 2. It increases the Activation energy of the reactant molecules. |
| 3. It increases the yield of chemical reactions. | 3. It cannot increase the yield of chemical reactions. |
| 4. It is also called the Promoter. | 4. It is also called Inhibitor. |
| 5. Example: Iron oxide acts as a positive catalyst in Haber’s process, increasing the yield of ammonia despite less nitrogen reaction. | 5. Example: The decomposition of hydrogen peroxide into water and oxygen is slowed by the use of acetanilide, which acts as a negative catalyst to slow the rate of hydrogen peroxide decomposition. |
Promoter or Accelerators
A substance that increases the catalyst activity is known as a promoter or accelerator.
Example: In Haber’s process, molybdenum or a mixture of potassium and aluminum oxides act as promoters.
Function of Promoters
The function of promoters is to serve a specific role during chemical reactions. The primary role of a promoter is to enhance the efficiency of a specific catalyst in a chemical reaction. Promoters play a crucial role in enhancing the activity of catalysts, thereby positively impacting the overall reaction. It is important to note that the only function that makes them more useful and essential during a chemical reaction is their promoter function.
A promoter, by itself, has little to no catalytic effect. Promoters have the ability to interact with the active sites of catalysts, resulting in significant modifications to the chemical effects of the catalyzed reaction.
Catalyst Poisons or Inhibitors
Substances that decrease the catalyst activity are known as catalyst poisons or inhibitors.
Example: In the hydrogenation of alkyne to an alkene, catalyst palladium is poisoned with barium sulfate in quinolone solution, and the reaction is stopped at the alkene level. This type of catalyst is known as Lindler’s catalyst.
Difference Between Catalyst Promotor and Catalyst Poison
The main distinction between a catalyst promoter and a catalyst poison lies in their impact on the effectiveness of the catalyst. While a catalyst promoter enhances the catalyst’s efficiency, a catalyst poison diminishes it. In Harber’s cycle, catalyst promoters such as molybdenum or a mixture of potassium and aluminum oxides are used. These promoters enhance the effectiveness of catalysts. On the other hand, carbon monoxide can act as a catalyst poison, specifically for platinum catalysts used in the oxidation of hydrogen.
Catalysis
When a catalyst is used to increase the rate of a chemical reaction, this phenomenon is known as catalysis.
Types of Catalysis
On the basis of nature and the physical state of the substance employed in the chemical reaction, catalysis is of three types:
- Homogeneous catalysis
- Heterogeneous catalysis
- Autocatalysis
Homogeneous catalysis
Homogeneous catalysis occurs when the reactants and the catalyst are in the same phase. Some examples of homogeneous catalysis are as follows:
- In the lead chamber process, sulfur dioxide is oxidized to sulfur trioxide with dioxygen in the presence of nitrogen oxides as the catalyst. All of the reactants, sulfur dioxide and oxygen, as well as the catalyst, nitric oxide, are in the same phase.
2SO2 (g) + O2(g) → 2SO3 (g) (in the presence of gaseous NO)
- H+ ions supplied by hydrochloric acid catalyze the hydrolysis of methyl acetate. The reactants and catalyst are both in the same phase.
CH3COOCH3 (l) + H2O(l) → CH3COOH (aq) + CH3OH (aq) (in the presence of aqueous HCl)
Heterogeneous catalysis
Heterogeneous catalysis refers to the catalytic process in which the reactants and catalysts are in different phases. The following are some examples of heterogeneous catalysis:
- In the presence of Pt, sulfur dioxide is oxidized to sulfur trioxide. The reactant is in a gaseous state, while the catalyst is solid.
2SO2 (g) → 2SO3 (g) (in presence of Pt(s))
- In Haber’s process, dinitrogen and dihydrogen combine to form ammonia in the presence of finely divided iron. The reactants are in a gaseous state, while the catalyst is solid.
4NH3 (g) + 5O2 (g → 4NO(g) + 6H2O(g) (in presence of Pt(s))
- Hydrogenation of vegetable oils with finely divided nickel as a catalyst. One of the reactants is in a liquid state, the other is in a gaseous state, and the catalyst is solid.
Vegetable oils(l) + H2 (g) → vegetable ghee (s) (in the presence of solid Nickel)
Autocatalysis
In the autocatalytic reaction, no specific catalyst is added. Instead, one of the products acts as a catalyst and increases the rate of formation of products.
Example: Decomposition of Arsene (AsH3) is formed by the Arsenic formed in the reactor as “autocatalyst”.
2AsH3 → 2As + 3H2
In this process, As acts as a catalyst.
Enzyme Catalysis
Enzyme catalysts or enzymes as a catalyst are biocatalysts that can be utilized in the transformation of organic compounds.
Enzymes catalyze a variety of reactions that occur in the bodies of animals and plants in order to sustain life. As a result, the enzymes are referred to as biochemical catalysts, and the phenomenon is known as enzyme catalysis.
Characteristics of Enzyme Catalyst
- A single enzyme catalyst molecule can convert up to a million molecules of reactant per second. As a result, enzyme catalysts are said to be extremely efficient.
- These biochemical catalysts are specific to specific types of reactions, which means that the same catalyst cannot be used in more than one reaction.
- A catalyst’s effectiveness is greatest at its optimum temperature.
Mechanism of Enzyme Catalyst
Enzymes contain surface cavities. These cavities have -COOH, -SH, etc. Biochemical particles have active centers. The substrate, which is oppositely charged to the enzyme, fits into a lock like a key. The products decompose due to complicated active groups. Therefore, two stages are needed:
First, combine enzymes and reactants:-
E+R→ER
Step two is disintegrating the complicated molecule to make the product.
ER→ E+R
A number of ways:
Proximity: Molecular solutions can be formed by enzymes. Transferring a phosphate group from ATP to glucose in a free solution has a very low likelihood of the two molecules coming close. ATP and sugar can collide with several molecules. ATP and sugar react better when they attach independently and tightly to an enzyme’s active site.
Orientation: When two molecules meet with enough energy to cause a reaction, the outcome varies. They must be oriented to transfer collision energy to the reactive link. Enzymes push reactive groups toward reactions by binding substrates.
Induced fit: There are several uses for enzymes. So, they differ from solid catalysts like metal catalysts employed in chemical hydrogenation. An enzyme’s conformation changes after attaching its substrates, stretching or deforming them to simulate the transition state. When bound to glucose, hexokinase clamshells. This structure makes substrates reactive.
The reactive amino acid groups: Side chains of amino acids include reactive residues. Histidine can absorb and/or supply substrate proton. Before hydrolysis, an acyl group might bind a serine side chain to water. Enzymes having specialized catalytic activity near a substrate speed up processes. A proton-coupled to histidine can immediately reach a substrate’s basic group.
Metal ions and coenzymes: Enzymes give reactive groups in addition to amino acid side chains. Biomolecules called coenzymes give chemical groups for catalysis. Like enzymes, coenzymes do not alter during catalysis. This distinguishes them from enzyme-transformed substrates like ATP.
- Coenzymes are not protein-based. The active areas of many enzymes contain metal ions and sometimes the substrate.
- Coenzymes provide chemical functional groups to proteins.
- Only sulfhydryl groups on amino acids can oxidize and reduce, therefore disulfide formation/breakage cannot affect most biomolecules’ functional groups.
- One of several coenzymes, usually NAD or FAD, is needed as electron acceptors and donors.
Examples of Enzyme Catalyst
- Hydrolysis of starch.
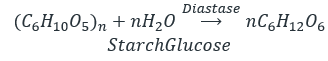
- Ethanol is obtained from glucose by the enzymatic action, glucose is converted into ethyl alcohol and CO2 by the enzyme catalysis of the zymase enzyme produced from yeast.
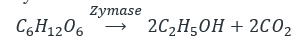
- Hydrolysis of urea
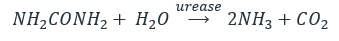
Video on Catalyst and Catalysis
References
- Gerlt J A. Protein engineering to study enzyme catalytic mechanisms. Curr Opin Struct Biol,1994, 4(4):593-600.
- https://www.creative-enzymes.com/resource/enzyme-catalysis_30.html#:~:text=Enzyme%20catalysis%20is%20a%20procedure,permanently%20conjugate%20with%20a%20cofactor.
- https://unacademy.com/content/jee/study-material/chemistry/mechanism-of-enzyme-catalysis/
- https://byjus.com/jee/catalyst/
- https://www.britannica.com/science/catalysis

