The carbylamine reaction, commonly called Hofmann’s isocyanide test, is a chemical test used to identify primary amines. This reaction involves the reaction of primary amine with alcoholic potassium hydroxide and chloroform to form isocyanide.
Interesting Science Videos
What is the carbylamine reaction?
When aromatic and aliphatic primary amines are heated with chloroform and alcoholic KOH, isocyanides or carbylamines are formed, and the reaction is known as a carbylamine reaction. The newly formed material has an unpleasant odor. The synthesis of carbylamine or isocyanide is easily recognized by its disagreeable odor.
In this reaction, a primary amine and chloroform are heated with alcoholic potassium hydroxide to produce alkyl isocyanide or carbylamine.
RNH2 + CHCl3 + 3 KOH → RNC + 3 KCl + 3H2O
The compound generated as product RNC is the alkyl isocyanide, and the reaction is known as an isocyanide test or Hoffmann’s carbylamine reaction. Secondary and tertiary amines are not affected by the carbylamine reaction.
Reaction mechanism of carbylamine reaction
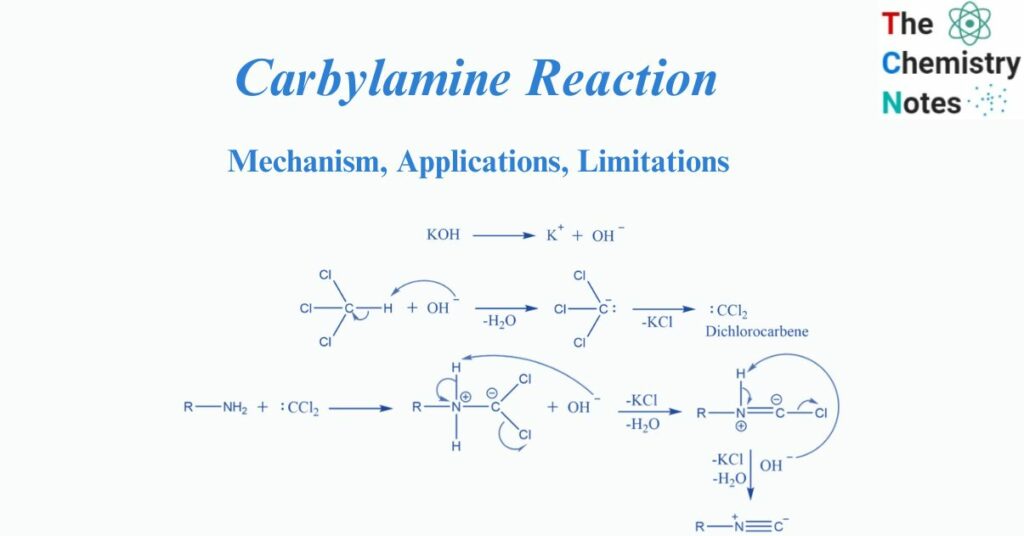
Chloroform undergoes dehydrohalogenation, producing the reactive intermediate dichlorocarbene.
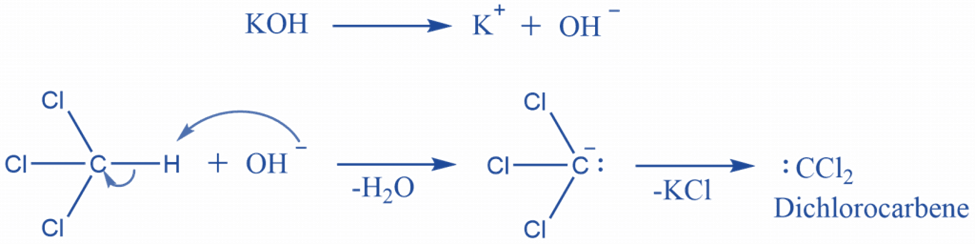
The electrophilic addition of dichlorocarbene to amine is followed by sequential dehydrochlorination in the presence of a base.
Hoffmann’s isocyanide test
Carbylamine reactions take place only with primary amines and not with secondary or tertiary amines. It is used to identify primary amine from secondary and tertiary amine, and the Carbylamine reaction in this context is known as Hoffmann’s isocyanide test. The test sample substance is placed in a test tube and heated with a base and chloroform in the presence of alcohol. When primary amines react with chloroform and base, a dichlorocarbene intermediate is formed, which leads to the creation of an isocyanide. This isocyanide molecule is distinguished by its characteristic odor. Secondary and tertiary amines do not emit this foul odor since they do not react with chloroform.
Why secondary and tertiary amines do not give carbylamine tests?
The carbylamine reaction is used to detect chloroform and a primary amine. This carbylamine test is used to differentiate between primary, secondary, and tertiary amines.
According to the mechanism, this is one of the best instances of an Alpha elimination reaction, in which the carbanion is generated in the first step and then loses the chloride Ion to form the highly reactive dichlorocarbene. The remaining two chlorine atoms will then be eliminated as HCL. Hydrogen is obtained from the nitrogen in amine for this purpose.
In regards to secondary and tertiary amines, nitrogen does not contain any more hydrogen atoms, and if these amines are reacted with chloroform, a sterically unstable end product is expected to form.
Applications of carbylamine reaction
- It is used for the detection of primary amines.
- It is used for the synthesis of isocyanide.
- The carbylamine reaction, also known as the Hofmann isocyanide test, is commonly used in laboratories to identify primary amines from secondary and tertiary amines.
- The produced isocyanides are utilized to make a variety of chemical compounds such as secondary amines, amides, and so on.
- The carbylamine reaction is widely employed in the fertilizer industry to produce isocyanides, which are then processed to yield a variety of different organic chemistry-based compounds.
Limitations of carbylamine reaction
- Secondary and tertiary amines do not give carbylamine reactions. So, it is only used for primary amines.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992
