Capillary electrophoresis (CE) is a highly sensitive separation technique that has been primarily developed based on the concept of high-performance liquid chromatography, thin-layer chromatography, and electrophoretic methods. The use of capillary electrophoresis (CE) has proven to be advantageous in the separation and analysis of bio-molecules and compounds with lower molecular mass. These substances, which are typically challenging to study using high-performance liquid chromatography (HPLC) or traditional methods of electromigration on slab gel, can be effectively separated using CE.
In recent times, conventional gel-based electrophoretic techniques have been superseded by high-performance capillary electrophoresis (HPCE), which is particularly well-suited for automation. The compound CE, which has received official recognition from various regulatory agencies including the FDA, is listed in the pharmacopeia and can be utilized for quantitative analysis. Capillary electrochromatography (CEC) is an emerging hybrid separation technique that combines the superior separation efficiency of capillary electrophoresis (CE) with high-performance liquid chromatography (HPLC).
Interesting Science Videos
What is Capillary Electrophoresis?
Capillary electrophoresis (CE), also known as capillary zone electrophoresis (CZE), is a highly efficient analytical technique employed for the separation of minute quantities of substances within a short period of time, yielding exceptional resolution. Using this methodology, it is possible to effectively segregate a total of 18 amino acids within a time frame of 16 minutes. The capillary electrophoresis principle is utilized for the sequencing and separation of significant biological macromolecules, such as proteins, peptides, DNA, RNA, and bio-polymers.
The analysis of materials at the nanolevel of 10−9 can be achieved using a capillary electrophoresis instrument with a detector sensitivity of 10−18. The principle underlying the separation of charged species is predicated on their ionic mobility or disparity in migration rate when subjected to an electric field. Cationic species of inorganic nature, such as lithium, sodium, potassium, and magnesium ions, can also be effectively separated through capillary zone electrophoresis (CZE).
Capillary Electrophoresis Principle
When an electric field of strength “E” is applied across a capillary, the speed with which an analyte moves in response to the electric field is a function of both its electrophoretic mobility and the electroosmotic mobility of the buffer.
The solute’s electric charge, molecule size, and shape, as well as the buffer’s ionic strength, pH, viscosity, and additives all play a role in the solute’s electrophoretic mobility. The following equation describes the electrophoretic velocity (Vep) of the solute:
Vep= μepE = (q/6πηr) (V/L)
where η is the viscosity of the electrolyte solution, V is the applied voltage, L= the length of the capillary, r is the Stoke’s radius of the solute, μep is its electrophoretic mobility, and q is its effective charge.
An electroosmotic flow is created when an electric field is delivered through a capillary that contains a buffer. Electrophoretic mobility is the determining factor in which speed. The properties of the buffer and the charge density of the capillary’s inner wall are what determine mobility. Here is the formula for electroosmotic velocity (Veo):
Veo = μeoE = (ε𝜁/η) (V/L)
where V is the applied voltage, μeo is the electrophoretic mobility, L is the length of the capillary, η is the viscosity of the electrolyte solution, and ε= the dielectric constant of the buffer and 𝜁= the zeta potential of the capillary surface.
The solute’s velocity (V) is given by: V= Vep+Veo
Depending on the solute’s charge, the analyte’s electroosmotic and electrophoretic mobility can be opposing. In capillary electrophoresis, anions move to oppose electroosmotic flow at slower speeds. Cations migrate in the same direction as electroosmotic flow at speeds greater than the velocity. In fact, electroosmotic conditions, cations, and anions are separated in the same run. t = l/ Vep+Veo = l(L)/ V(Vep+Veo) for the solute to migrate from the injection end of the capillary to the detection point (effective capillary length).
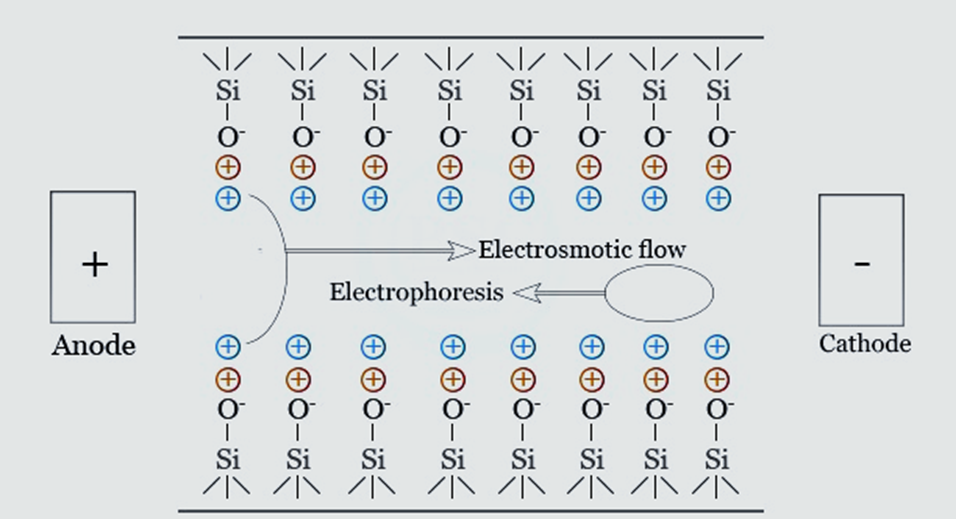
Uncoated, negatively charged fused silica capillaries are commonly employed in applications where electroosmotic flow flows from anode to cathode. The electro-osmotic flow must be held constant so that the solutes’ migration speed can be replicated from one experiment to the next. It may be necessary to minimize or remove electroosmotic light for some experiments by adjusting the capillary’s inner wall or the buffer solution’s concentration, composition, or pH.
Each analyte ion generates a zone after adding the sample. Background electrolyte migration creates the zone. Different events propagate each solute band. The zone expands only due to solute molecular diffusion in the capillary under ideal conditions. The zone’s efficacy is calculated as the number of theoretical plates (N):
N= (μep+μeo) (Vl)/ 2DL
Where D is the molecular diffusion coefficient of the solute buffer.
In practice, band dispersion also depends on a number of other factors, including the length of the injection plug, the size of the detector cell, the unevenness of the buffer reservoirs, the conductivity mismatch between the sample and the buffer, the adsorption of the sample onto the capillary wall, and the dispersion of heat. The separation of two bands is accomplished by optimizing the efficiency of each analyte in that band and changing the electrophoretic mobility of the analytes and the electroosmotic mobility induced in the capillary.
Rs= N(μepb-μepa)/ 4(μaep+μeo);
where μepa and μepb= the two analytes’ electrophoretic mobilities, μaep is the average electrophoretic mobility of the two analytes calculated by: μaep= ½ ( μepb+μepa).
Types of Capillary Electrophoresis
- Capillary zone electrophoresis (CZE): In capillary zone electrophoresis (CZE), simply a buffer is present in the capillary. The electrophoretic mobility and electroosmotic flow of the analyte determine the velocity of the band through which it travels. Substances having molecular weights between 2,000 and 100,000 can be analyzed well using this form of electrophoresis.
- Capillary Gel Electrophoresis (CGE): Similar to gel electrophoresis, the separation in capillary gel electrophoresis takes place in a capillary filled with a gel functioning as a molecular sieve. The separation of molecules occurs on the basis of their molecular weight. Smaller molecules migrate more rapidly than larger ones because they can more easily navigate the gel network. Proteins, DNA fragments, and other biological macromolecules with similar charge-to-mass ratios can be separated by size using capillary gel electrophoresis.
- Micellar Electrokinetic Capillary Chromatography (MEKC): In MEKC, a surfactant is present in an electrolytic solution above the critical micellar concentration, creating a hybrid between electrophoresis and chromatography. Using the partition coefficient between the micelle-based pseudo-stationary phase and the aqueous buffer, both neutral and charged solutes can be evenly distributed using this method.
- Capillary electrochromatography (CEC): Capillary electrochromatography combines capillary electrophoresis and chromatography, notably HPLC. Electrophoretic mobility or partition ratios separate analytes. Electroosmotic force moves the mobile phase across the chromatographic bed. CEC outperforms HPLC and capillary electrophoresis. It separates amino acids, proteins, peptides, and carbohydrates enantiomerically. It is mostly utilized in pharmaceutical and polymer analysis industries to distinguish acidic and basic medicines.
- Capillary isoelectric focusing (CIEF): Using ampholytes dissolved in a separation buffer to provide a pH gradient, CIEF allows for the migration of charged molecules. This method is typically used for enantiomeric separations and the separation of amino acids, proteins, peptides, and carbohydrates, and consists of three stages: loading, focusing, and mobilization.
- Capillary Isotachophoresis (CITP): Ions in a flowing buffer with a continuous and quickly diminishing conductivity gradient are separated electrophoretically for CITP. The separation of tiny ions, such as DNA and other biological macromolecules, is its primary use.
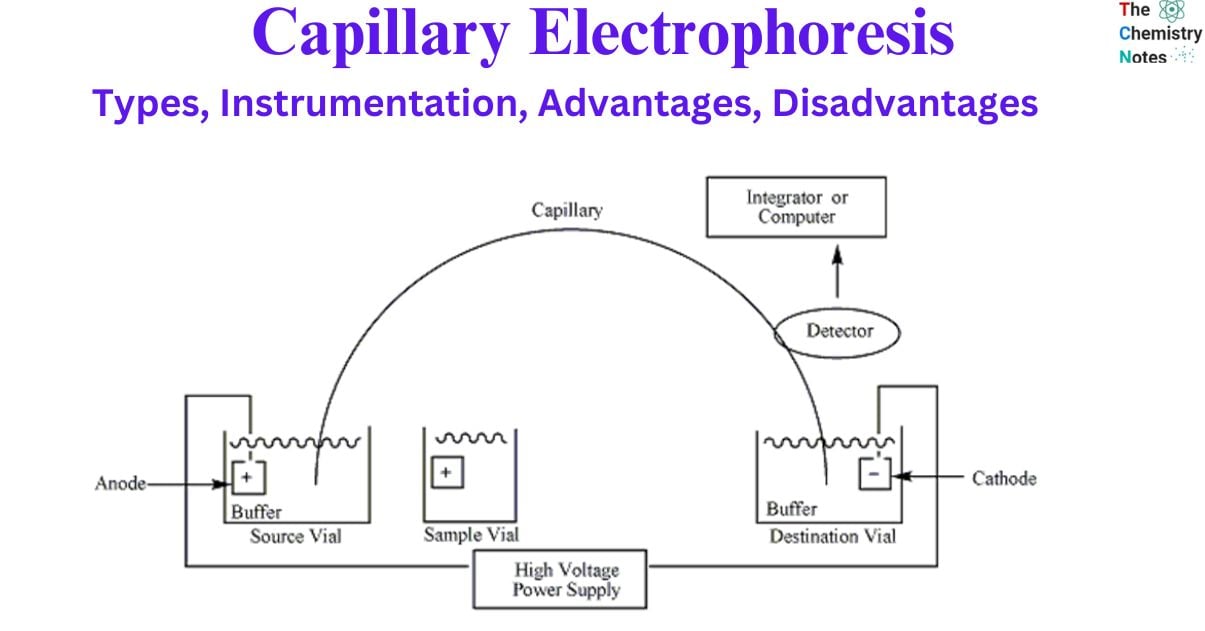
Capillary Electrophoresis Instrumentation
Capillary electrophoresis (CE) is a highly effective analytical technique for the separation and analysis of a wide range of substances in the lab. Having the proper equipment and environment is essential for a successful CE. In this article, we’ll provide an overview of the standard capillary electrophoresis equipment set.
- Power supply: A high-voltage, variable-current power supply is the foundational component of CE equipment. The electric field used in sample separation is produced by the power source.
- Buffer reservoirs: CE requires two identical buffer reservoirs filled with anodic and cathodic solutions. The stability of the electric field depends heavily on these buffer reservoirs.
- Electrodes: A cathode and an anode, both of which need to be submerged in the buffer reservoirs, are necessary for the CE process. The electric field is produced by the electrodes, which are linked to the power source and are an essential component in the process.
- Capillary: Fused silica is used to construct the capillary that is utilized in CE, and its diameter is typically less than one hundred microns. The capillary functions as a route for the samples to pass through on their way to being separated.
- Optical viewing window: CE involves the utilization of an optical viewing window that is in proper alignment with the detector. The viewing window makes it possible to observe the separation process while it is taking place.
- Injection system: An appropriate injection system is needed for loading sampless and buffers into the capillary during CE. The injection can be automated for increased accuracy. Samples are often injected using one of three methods: gravity, pressure or vacuum, or electrokinetics.
- Detector: Conductimetric, fluorimetric, absorption spectrophotometry (UV and visible), amperometric, or mass spectroscopic detectors are all suitable for monitoring the amount of substance passing through the capillary at any given time in CE.
- Thermostatic system: Thermostatic control of the capillary environment is essential for CE. The reliability and precision of the outcomes are guaranteed by the controlled temperature.
- Recorder: In CE, the data collected during electrophoresis must be recorded for further analysis. The data must be recorded so that it can be analyzed later.
- Computer or suitable integration: In addition, CE requires a suitable integrator or computer for digital data conversion. It is easier to analyze and evaluate the digital data.
Procedure for Capillary Electrophoresis
In order to preserve the resolution, two procedures are employed to introduce a micro-volume of sample that does not exceed 1% of the effective length of the capillary.
- Hydrostatic injection: To do this, a capillary is dipped into the sample solution (including an electrolyte) while a small vacuum is created at the other end. Applying pressure to the sample solution at a level of about 50 mbars can enhance the procedure.
- Electro-migration injection: This method is employed in gel electrophoresis, and it entails slightly immersing the capillary in a sample that has been placed at a potential with the suitable polarity relative to the other extreme. This manner of injection, in contrast to the traditional method, causes a discriminating action on the chemicals present, resulting in a non-representative sample composition.
Since injection loops are not available for volumes between 5 and 50 nL, the accuracy of the hydrostatic injection method is inferior to that of HPLC. The flow rate F in a tube (radius r, length L) for a liquid with a dynamic viscosity depends on several of the factors that appear in the well-known Poiseuille expression. An approximation of the capillary’s “entering flow rate” can be calculated. This correlation shows that a capillary’s volume will increase by a factor of 16 if its radius is doubled. The volume is related to the pressure gradient P. The opposite occurs when the capillary length L is increased. Quantitatively, HPCE would benefit from the usage of an internal standard.
CE’s analyte detection techniques are remarkably close to those of liquid chromatography. There are just three major approaches presented here.
- Direct UV/Vis detection: Typically, a diode array detector is used for the detection of UV-visible absorbance. In order to detect the amount of UV light passing through the capillary, the polyimide covering it must be burned away, forming a tiny window. Due to the fact that the detector cell is integral to the capillary, this setup wastes extremely little space. When applied at short wavelengths, UV is at its most sensitive.
- Fluorescence detection: For this detection method to work, the analytes must be chemically labeled with a fluorophore, a process known as derivatization. The next step is the standard separating procedure. When analytes pass through a detection window, the light emitted by the fluorophores causes them to change wavelength. Using a powerful laser source improves the procedure’s sensitivity.
- Mass spectrometry detection: When coupled with a CE instrument, a mass spectrometer can offer data on the molecular masses of solutes and the structures of those solutes, both of which can aid in the identification of unknowns. However, it is not simple to connect the two devices. Therefore, in order to acquire gas phase ions of the solutes, an additional flow of liquid must be added to the CE eluent. Biochemical analysis makes extensive use of MS detection.
- Indirect UV detection: This technique is employed for inorganic ionic analytes, which are not affected by ultraviolet light. Capillary buffers with high absorption coefficients, such as chromates, and phthalates, are used for this purpose. Due to the exclusion of the UV-absorbing buffer, the amount of light passing through the capillary rises as the analytes go past the detector, resulting in negative peaks.
How does Capillary Electrophoresis Work?
- The CE system’s capillary is formed of fused silica, and its inner layers are negatively charged.
- Because of differences in ionic mobility and interaction with the liquid phase or electrolyte medium, molecules in a mixture begin to separate when a high voltage is applied at the capillary’s end.
- Smaller molecules or ions with greater charges travel through the molecules at a faster rate than larger molecules or ions with lower charges.
- By generating a pH or conductivity gradient in the electrolyte solution, the molecules can be concentrated.
- After the molecules have been separated, they are picked up by a detector and shown on the screen as independent peaks in relation to the migratory times.
- The charge, radius, and solvent viscosity of molecules all play a role in their electrophoretic mobility in reaction to an electric field.
It is possible to study tiny compounds with great sensitivity by combining capillary electrophoresis and mass spectrometry (a technique pioneered by Richard D. Smith). The technique’s high-resolution separation method and efficiency make it preferable to others, such as High-performance liquid chromatography (HPLC).
Advantages of Capillary Electrophoresis
The use of microcapillaries in capillary electrophoresis confers several benefits over conventional electrophoresis and chromatography. Some of the major advantages include:
- Highly efficient: Using a capillary with a narrow diameter allows for effective heat transfer. It has been argued that capillary electrophoresis is just as effective as liquid chromatography.
- High resolution: CE is capable of performing highly accurate ion and molecule separations based on size, charge, and hydrophobicity. Because of this, precise and comprehensive findings are within reach.
- High selectivity: CE’s excellent detection sensitivity makes it possible to examine and detect compounds in minute concentrations.
- Versatility: Ions, tiny molecules, proteins, and DNA/RNA are just a few of the many compounds that can be analyzed with CE.
- Less time-consuming: It doesn’t take long to read the result of the separation if the reader is conversant with computer software. Also, from beginning to end (setup to separation), the process takes just one hour. Therefore, there are more benefits to capillary electrophoresis than there are to other separation methods.
Application of Capillary Electrophoresis
- The capillary zone electrophoresis principle allows for the separation and analysis of large biological molecules like DNA, mRNA, protein, peptide, and biopolymers.
- Capillary electrophoresis is a widely employed technique in the field of forensic science for the purpose of detecting and analyzing DNA fragments. The utilization of a capillary electrophoresis instrument holds significant importance and offers a cost-effective approach in the context of DNA sequencing. The technology offers a high level of precision in the process of sequencing data.
- One significant application of capillary electrophoresis instruments in the field of forensic biology involves the identification and analysis of mRNA molecules. This enables forensic biologists to determine the origin of biological fluids or tissues present in forensic samples.
- Affinity capillary electrophoresis (ACE) represents a modified variant of capillary electrophoresis. Affinity capillary electrophoresis instruments have been employed by pharmaceutical companies for drug detection and analysis owing to their cost-effectiveness and superior resolution capabilities.
- This method can be employed for the concurrent analysis and isolation of inorganic ions, such as ammonium (NH4+), sodium (Na+), potassium (K+), magnesium (Mg2+), and calcium (Ca2+) ions. This form of segregation can be executed with a high degree of precision within a brief time frame.
- Capillary electrophoresis is employed in the analysis of diverse food categories, including fermented food and beverages, for the purpose of detecting and characterizing pigments, flavonoids, carbohydrates, vitamins, proteins, and colorants.
- Standards solutions of small molecules in pharmaceuticals are prepared and analyzed using capillary electrophoresis. In clinical laboratories, CITP is essential for identifying cholesterol levels for lipid profile analysis.
References
- Robert, F., Bouilloux, J. P., & Denoroy, L. (1991). L’électrophorèse capillaire: principe et applications [Capillary electrophoresis: principle and applications]. Annales de biologie clinique, 49(3), 137–148.
- Coşkun, Ö., & Öztopuz, Ö. Electrophoresis Applications Used in Medicine. Medical Sciences, 15(1), 12-25.
- Baker DR (1995). Capillary Electrophoresis. New York: John Wiley & Sons, Inc.Skoog DA, Holler FJ, Crouch SR (2007). Principles of Instrumental Analysis (6th ed.). Belmont, CA: Thomson Brooks/Cole Publishing.
- Borst, C., Belal, F., & Holzgrabe, U. (2013). Possibilities and limitations of capillary electrophoresis in pharmaceutical analysis. Die Pharmazie, 68(7), 526–530.
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumentation_and_Analysis/Capillary_Electrophoresis#:~:text=Capillary%20electrophoresis%20is%20an%20analytical,viscosity%2C%20and%20the%20atom’s%20radius.
