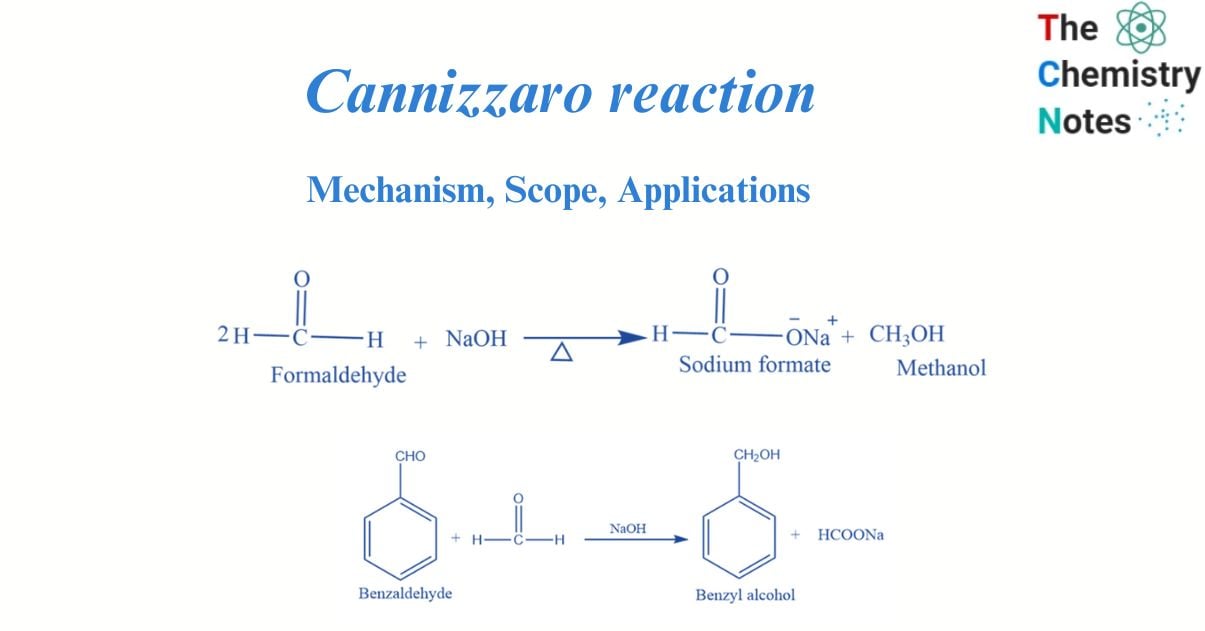
Cannizzaro reaction is a chemical process in which an aldehyde without a hydrogen atom in the alpha position undergoes base-induced disproportionation.
Cannizzaro Reaction occurs when one molecule of aldehyde is reduced to the corresponding alcohol and another is oxidized to the salt of the corresponding acid. When Cannizzaro utilized potash to separate benzyl alcohol and potassium benzoate from benzaldehyde (potassium carbonate), he first accomplished this transition. Sodium hydroxide or potassium hydroxide is typically used in the procedure, generating the sodium or potassium carboxylate salt of the carboxylic-acid product.
What is Cannizzaro’s reaction?
Aldehydes without the alpha hydrogen when heated with concentrated NaOH undergo a disproportionation reaction. In this reaction, one-half of the aldehyde oxidized to carboxylic acid while another half reduced to form alcohol.
The Cannizzaro reaction is an organic reaction in which an aldehyde without active hydrogen performs a redox reaction under the influence of a strong base. Vanillin, benzaldehyde, syringaldehyde, and formaldehyde are examples of aldehydes that do not contain active hydrogen. Under the action of a strong base (NaOH), they undergo intramolecular and intermolecular oxidation-reduction processes to generate a molecule of carboxylic acid and a molecule of alcohol.
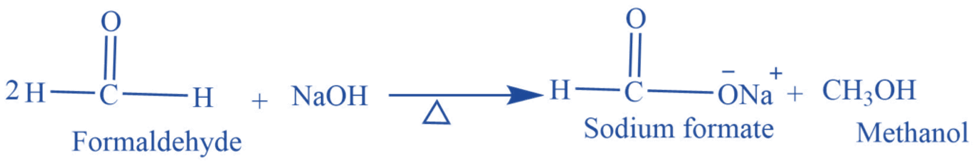
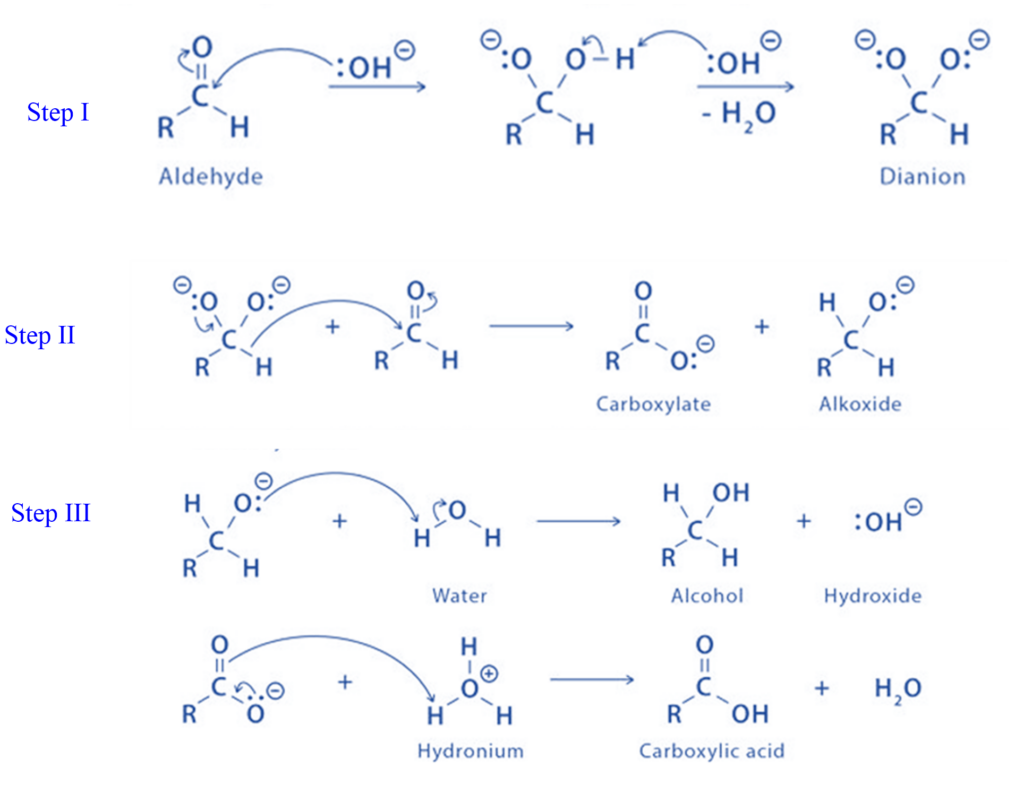
Mechanism of Cannizzaro reaction
- Step I: Hydroxide ions’ nucleophilic attack on the carbonyl group results in the formation of the intermediate.
- Step II: The hydride ion is transported directly from the intermediate formed in Step 1 to another aldehyde molecule’s electron-deficient carbonyl carbon atom.
- Step III: The intermediate generated in step two, the alkoxide ion, and the carboxylic acid rapidly exchange protons to give final products.
Scope of Cannizzaro’s Reaction
- Aldehydes with an alpha hydrogen atom have been decongesting due to strongly alkaline reactions, leading to enolates and potentially aldol reactions. Because it takes two aldehydes to make one acid and one alcohol, the method only produces 50% alcohol and carboxylic acid under ideal circumstances.
- To prevent low yields, the cross-Cannizzaro reaction, which combines aldehyde with a much more desirable molecule, can be carried out more frequently.
- The reducer in this form is formaldehyde, which is oxidized to sodium formate while alcohol is changed into another aldehyde molecule. In this case, one aldehyde can be converted to its matching product rather than losing half of a single reactant for each of the two products. The output of the precious chemical is therefore high even though the atomic economy is still poor.
Cross Cannizzaro reaction
When formaldehyde and benzaldehyde are reacted with caustic soda, benzyl alcohol, and sodium formate are produced. This reaction is commonly called cross Cannizaro reaction. It happens when an oxidant aldehyde in a Cannizzaro reaction is different from the reductant aldehyde. In this process, benzaldehyde is reduced to the corresponding alcohol while formaldehyde is oxidized to formic acid.
There is no electron donating groups on formaldehyde, which makes the first nucleophilic addition of the hydroxide anion faster on formaldehyde.
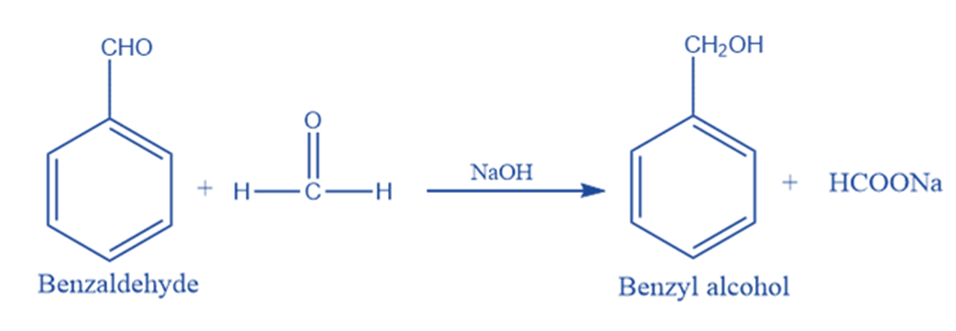
The quantitative reduction of some aldehydes may be accomplished by using the preferred oxidation of formaldehyde in crossed Cannizzaro reactions. Finally, a non-enolizable aldehyde can be disproportionated using the Cannizzaro process. The Cross Cannizzaro reaction is employed to improve the precious product’s yield.
Mechanism of Cross Cannizzaro reaction
A nucleophile, such as a hydroxide ion, attacks the carbonyl group of the provided aldehyde, which causes a disproportionation reaction and the creation of an anion with two negative charges. This intermediate is now suitable for usage as a hydride-reducing agent. Due to its instability, the intermediate releases a hydride anion.
This hydride anion attacks another aldehyde molecule. The doubly charged anion now becomes a carboxylate anion, while the aldehyde has converted into an alkoxide anion. In this final stage, water donates a proton to the alkoxide anion, resulting in the final alcohol product. Alkoxide is more basic than water, which allows the reaction to continue. The carboxylate ion is now capable of producing the ultimate carboxylic acid product when acid treatment is applied.
Step I: First, a dianion is produced by a nucleophilic attack on the carbonyl group.
Step II: The dianion is changed into a carboxylate and another aldehyde molecule is transformed into an alkoxide ion.
Step III: Protonation of the carboxylate into a carboxylic acid and the alkoxide into an alcohol.
Applications of Cannizzaro reaction
- The intramolecular Cannizzaro reaction of phthalaldehyde results in (o-hydroxymethyl) benzoic acid.
- Intermolecular Cannizzaro reactions can change -keto aldehydes into -hydroxycarboxylic acids.
- In the presence of strong alkali, furfural produces furoic acid and furfuryl alcohol.
- The Cannizzaro reaction can be utilized to influence a non-enolizable aldehyde disproportionation.
- In the industry, polyols are produced utilizing a combination of the crossed Cannizzaro reaction and aldol condensation. Polyols are highly valuable and used in a variety of industrial processes.
- Neopentyl glycol is a component of polyesters that is used to create plasticizers, varnish coatings, synthetic lubricants, and resins for use in or on board aircraft. Neopentyl’s chemical makeup renders it resistant to heat, light, and hydrolysis.
- Pentaerythritol is in high demand as a raw ingredient in the varnish industry. In goods with an increased fatty acid content, pentaerythritol esters are utilized as oil additives, plastifying agents, and emulsifiers.
- Trimethylolpropane is employed as a glycerin alternative in a number of processes, such as the production of polyesters, polyurethanes, and alkaline resins.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry: reactions mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. roduction of polyesters, polyurethanes, and alkaline resins.
