Different molecules or compounds are formed based on the fundamental structural properties. The bond angles is one such measure. It is an important characteristic because if the angle between the bonded electrons or atoms is improper, the atom becomes unstable and cannot live in its surroundings. As a result, when different atoms make bonds, they are not clearly obtaining stability if the angles are not fulfilled.
Bond angle is the angle formed by two bonds, i.e. the angle formed by two orbitals that contain a pair of bonding electrons around the central atom in a complex molecule or an ion. This angle is normally measured in degrees and then determined using the spectroscopic approach.
Interesting Science Videos
Bond Angles and VSEPR Theory
- Bond angles are investigated using the valence shell electron pair repulsion (VSEPR) hypothesis.
- This theory predicts the shape of a molecule based on the number of bonding and lone pairs.
- According to this theory, the lone pairs in the core atom’s valence shell will restructure themselves to reduce repulsion and increase distance between them.
- As a result, the molecule adopts a constant geometric form.
- The bond angle is determined by the angle created by the surrounding bonds.
Bond Angle and Molecular Shapes
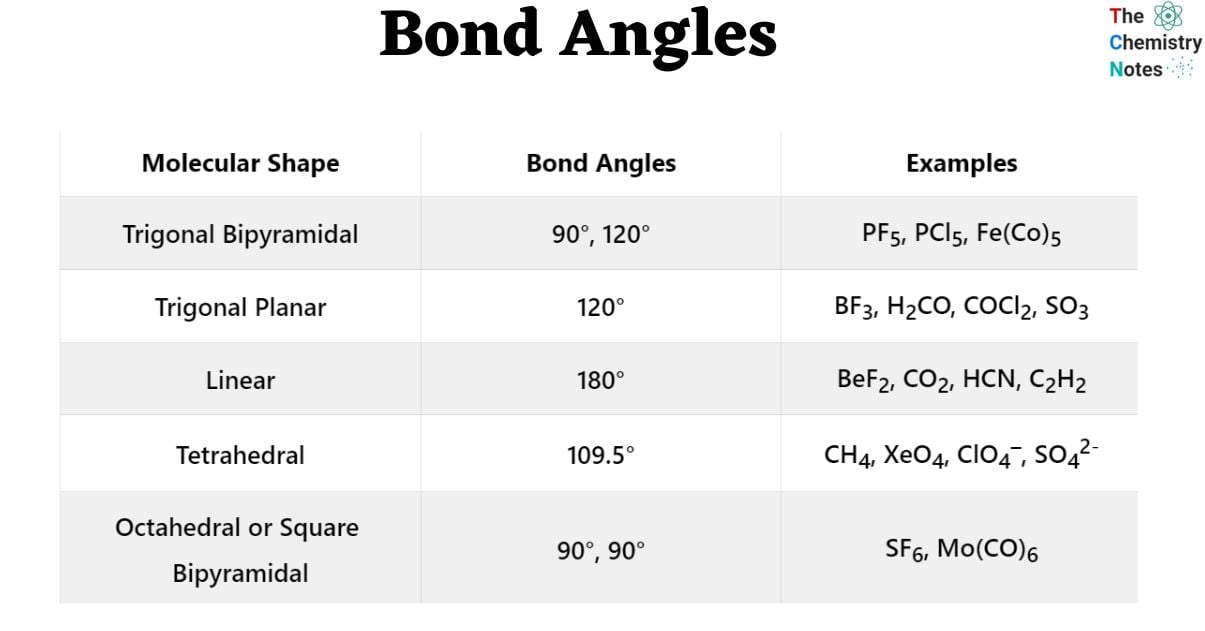
The VSEPR theory predicts five basic geometries of molecules that have no lone pairs. These five structures may be distinguished by bond angles, as illustrated in the table below.
| Molecular Shape | Bond Angles | Examples |
|---|---|---|
| Trigonal Bipyramidal | 90°, 120° | PF5, PCl5, Fe(Co)5 |
| Trigonal Planar | 120° | BF3, H2CO, COCl2, SO3 |
| Linear | 180° | BeF2, CO2, HCN, C2H2 |
| Tetrahedral | 109.5° | CH4, XeO4, ClO4–, SO42– |
| Octahedral or Square Bipyramidal | 90°, 90° | SF6, Mo(CO)6 |
Angles of Bond
Bond angle is expressed in degrees and is further quantified by the spectroscopic method.
This information is helpful in defining the structure of a molecule in addition to giving an accurate image of the distribution of bound electron pairs around the atoms. It also gives a hint as to how the bonded electron pairs around the atoms are distributed, which is crucial for determining the molecular structure.
Factors That Effects Bond Angles
Hybridization
The bond angle of the central atom is dictated by its state of hybridization. The bond angle grows in direct proportion to the s character’s size.
- sp:180 degrees
- sp2: 120 degrees
- sp3:109°28′
Lone Pair Repulsion
The existence of a lone pair of electrons at the central atom influences the molecule’s bond angle. When the lone pair tries to reject the bond pair it is attempting to repel, the bond angle drops. As a result, the bond angles of certain compounds are affected by the quantity of lone pairs.
- For example, the bond angle of CH4 (which includes no lone pair) is 109°, but the bond angle of NH3 (which has one lone pair) is 107°.
Electronegativity
As the electronegativity of the central atom diminishes, so does its bond angle.
- For example, NH3 has a bond angle of 107°, whereas PH3 has a bond angle of 93.5°.
Effect of Lone Pairs on Bond Angle
The molecule structure is influenced by lone pairs of electrons on the central atom. The lone pairs are placed in the core atom’s atomic orbitals and resist the bond pairs, creating a deviation from the previously stated geometry. As a result, certain compounds’ bond angles rely on the number of lone pairs.
To understand this impact, let’s look at the tetragonal form. A tetrahedral-shaped molecule with a conventional bond angle of 109.5° requires four electron pairs (bonding and nonbonding). Let us see how the form of methane, ammonia, and water changes.
- Methane doesn’t have lone pairs. Thus, it has a tetrahedral form with a conventional bond angle of 109.5°.
- Ammonia (NH3) is made up of three bond pairs and one lone pair. The bond angle will be reduced to 107° by the lone pair, which will force the bond pairs apart. The resulting molecular form is trigonal pyramidal.
- There are two bond pairs and two lone pairs in water (H2O). The bond angle will be reduced to 104.5° as a result of the two lone pairs further repelling the bond pairs. The molecule’s overall shape is bent or angular.
Video on Bond Angles
References
- https://laney.edu/pinar-alscher/wp-content/uploads/sites/219/2016/04/ws-7-bond-angles.pdf
- https://chemistrytalk.org/molecular-geometry-and-bond-angles/
- https://www.chemistrylearner.com/bond-angles.html
- https://unacademy.com/content/jee/study-material/chemistry/bond-angle/

