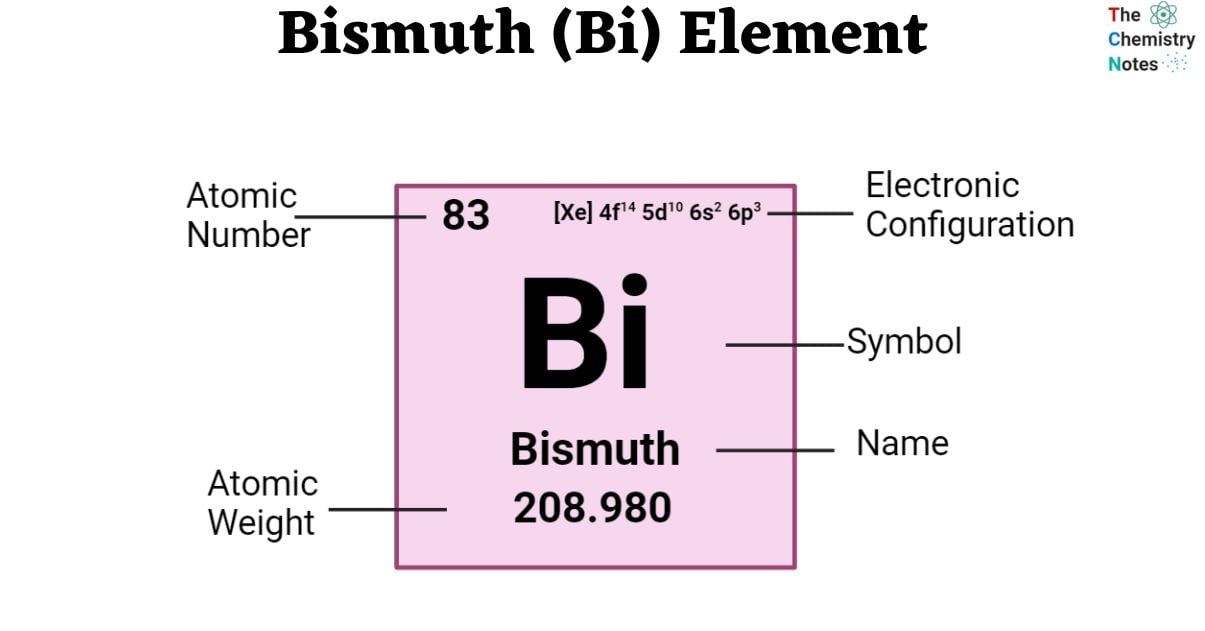
Bismuth is a chemical element with an atomic number of 83 and is represented by the symbol ‘Bi’ in the periodic table. It is white with a reddish pink tinge in appearance classified as a post-transition metal and belongs to the p-block of group 15 of the periodic table. Post-transition metals have certain properties in common with transition metals, but they are softer and conduct less well. Bismuth’s electric and thermal conductivity is, in fact, extraordinarily low for a metal.
Bismuth is roughly twice as plentiful in the Earth’s crust as gold. Bismuthinite and bismite are the most significant bismuth ores. Bismuth is most frequently found in a +3 oxidation state, however, it can also occur in a +5 state, also found in +2 and -3 oxidation states, which is quite uncommon. Bismuth +5 compounds like BiF5 and NaBiO3 are well-known, but their synthesis is challenging. Bismuth crystals are very beautiful because the various levels of oxidation disrupt the light wavelengths that reflect on their surface, producing a genuine rainbow of colors.
Interesting Science Videos
History of Bismuth
- The element is believed to have been discovered in 1400 AD by an unnamed alchemist.
- Despite its use as an alloying component in cast printers and caskets, Georgios Agricola, a German mineralogist, classified bismuth as a sort of metal with distinct qualities in the early 1500s.
- Caspar Neuman, a German professor, conducted the same analysis as a German mineralogist in the early 1700s.
- Finally, in 1753, the French scientist Claude-François Geoffroy provided additional evidence indicating the presence of a new metal known as bismuth.
- The name derives from ‘Bisemutum’ which is a variation of the German term ‘Weise Masse’ meaning ‘White Mass’.
Occurrence of Bismuth
- Bismuth is roughly twice as plentiful in the Earth’s crust as gold. Bismuthinite (Bi2S3) and bismite (Bi2O3) are the most significant bismuth ores.
- Bismuth occurs naturally as the metal itself and as crystals in the sulfide ores of nickel, cobalt, silver, and tin.
- Bismuth is mostly generated as a by-product of lead and copper smelting, particularly in the United States.
- It is mined mostly in Bolivia, Peru, Japan, Mexico, and Canada, although only to the amount of 3.000 metric tons each year.
Isotopes of Bismuth
Bismuth contains 33 isotopes with mass values ranging from 185Bi to 217Bi. Only 209Bi is adequately stable.
| Isotope | Natural Abundance (% atoms) |
|---|---|
| 209Bi | 100 |
Despite the fact that 209Bi is radioactive, its half-life is 1.9 x 1019 years and its alpha particle disintegration is extraordinarily sluggish. If exactly 100 grams of 209Bi existed at the beginning of the universe 14 billion years ago, the cosmos would still contain 99.9999999 grams of it now.
Elemental Properties of Bismuth
| Electronic Configuration | [ Xe ] 4f14 5d10 6s2 6p3 |
| Atomic Number | 83 |
| Atomic Weight | 208.9804 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 15, 6, p-block |
| Density | 9.80 g/cm3 at 20 °C |
| Appearance | silvery with pinkish tinge |
| Van der Waals radius | 0.152 nm |
| Electron shells | 2, 8, 18, 32, 18, 5 |
| Electrons | 83 |
| Protons | 83 |
| Neutrons in the most abundant isotope | 126 |
Physical Properties of Bismuth
- Bismuth has an atomic number of 83 and is a white with pinkish hint in appearance belonging to the post-transition group of metal. It has a melting point of 271.5 °C (520.7 °F) and a boiling point of 1564 °C (2847 °F).
- Bismuth is one of the few elements that is denser in liquid form than in solid form. It has a solid phase density of 9.78 g/cm3 and a liquid or molten phase density of 10.05 g/cm3.
- Bismuth is the most naturally diamagnetic of all metals, which means it resists magnetization and is repelled by magnetic fields.
- Bismuth also has an extraordinarily high electrical resistance for a metal.
- Bismuth has poor heat conductivity. It has the lowest heat conductivity of any metal except mercury.
- Bismuth has the peculiar feature of expanding when it freezes, similar to water. Other elements that expand when frozen include silicon, gallium, antimony, and germanium.
- In normal conditions, bismuth has a rhombohedral lattice structure. However, compression changes its structure so it is monoclinic at 2.55 GPa, tetragonal at 2.7 GPa, and body-centered cubic at 7.7 GPa.
| Color/physical appearance | metallic, white-reddish pink |
| Melting point/freezing point | 544.7 K (271.5 °C, 520.7 °F) |
| Boiling point | 1837 K (1564 °C, 2847 °F) |
| Density | 9.80 g/cm3 at 20° |
| Malleability | No |
| Ductility | No |
| Electronegativity | 2.02 (Pauling Scale) |
Chemical Properties of Bismuth
- The metal is resilient in oxygen and water. It does not change color when exposed to air and generates an insoluble compound when immersed in water.
- At boiling point, the element oxidizes fast and creates an oxide covering. This coating is a bright yellow.
- Bismuth interacts with halogens to create bismuth (III) halides. It also forms a +5 oxidation state compound with fluorine, bismuth(V) fluoride.
- While this element is rather inert, it interacts strongly with both dilute and concentrated nitric acid. When bismuth (III) nitrate, nitric oxide, and water are mixed, they generate bismuth (III) nitrate. It is sensitive to hydrochloric acid, nitric acid, and sulfuric acid.
Chemical Reaction of Bismuth
- The Reaction of Bismuth with Air
When heated, bismuth interacts with oxygen in the air to generate bismuth (III) oxide, Bi2O3. The flame is blue-white.
4 Bi (s) + 3 O2 (g) → 2 Bi2O3 (s)- The Reaction of Bismuth with Water
Bismuth interacts with water at high temperatures to generate the trioxide bismuth (III) oxide, Bi2O3.
2 Bi (s) + 3 H2O (g) → Bi2O3 (s) + 3 H2 (g)- The Reaction of Bismuth with Halogens
Bismuth combines with fluorine, F2, to generate bismuth (V) fluoride pentafluoride.
2 Bi (s) + 5 F2 (g) → 2 BiF5 (s) [white]Bismuth combines with the halogen fluorine, F2, to generate bismuth (III) fluoride under regulated circumstances.
2 Bi (s) + 3 F2 (g) → 2 BiF3 (s) [gray]Bismuth interacts with the halogen chlorine, Cl2, to generate bismuth (III) chloride under regulated circumstances.
2 Bi (s) + 3 Cl2 (g) → 2 BiCl3 (s) [yellow]Bismuth combines with the halogen bromine, Br2, to generate bismuth (III) bromide under regulated circumstances.
2 Bi (s) + 3 Br2 (g) → 2 BiBr3 (s) [yellow]Bismuth combines with the halogen iodine, I2, to generate bismuth (III) iodide under regulated circumstances.
2 Bi (s) + 3 I2 (g) → 2 BiI3 (s) [red to gray]- The Reaction of Bismuth with Acids
Bismuth dissolves in concentrated sulfuric acid, H2SO4, generating SO2, nitric acid, HNO3, and hydrochloric acid, HCl. Bismuth (III) chloride is formed when hydrochloric acid is combined with oxygen.
4 Bi (s) + 3 O2 (g) + 12 HCl (aq) → 4 BiCl3 (aq) + 6 H2O (l)Uses of Bismuth
Bismuth is a versatile element that finds use in medications, cosmetics, lead substitutes, metals, and synthetic fibers. Some important uses of bismuth are listed below:
- In the treatment of nausea, heartburn, and upset stomachs, bismuth subsalicylate, an insoluble salt of trivalent bismuth and salicylic acid, is utilized.
- Bismuth nitrates are extensively used as pigments and are the starting ingredient for bismuth vanadate colors. Bismuth vanadate is a brilliant yellow solid that is nontoxic, stable, and weather-resistant. These features make it perfect for architectural and industrial applications, coils, and powder coatings. It’s also a fantastic concealing powder that conceals the surface of an object.
- Bismuth is regarded as a “green” element. This metal is non-toxic in the environment and poses no industrial hazards. Shotgun shells composed of this material are used in reloading hunting revolvers and rifles because they are less expensive than lead and tungsten ammunition with similar properties. To avoid lead pollution in rivers used for bird hunting, it typically replaces the lead found in shotgun ammunition.
- Because Bi has a high electrical resistance, its oxide nanoparticles are utilized as cathodes in solid fuel cells and in cancer imaging.
- Adding bismuth to an alloy lowers the melting temperature. Low-melting alloys are frequently used in heat-resistant electronics such as fire sprinklers, fire alarms, and boiler plugs. Furthermore, while transitioning between a liquid to a solid form, bismuth increases in volume by 3.3%. When applied to an alloy, it prevents solidification and shrinkage. This allows it to compensate for its incapacity to be reused.
- Bismuth-electroplated baths are essential in the production of bearings for gasoline and diesel engines.
- In the casting of mirrors, reflectors, and lights, bismuth bronze—an alloy of copper, zinc, nickel, lead, and bismuth—is utilized.
Health Effects of Bismuth
- Bismuth and its salts can cause renal damage, however, the severity is usually mild. Large quantities are fatal. It is recognized as one of the least harmful heavy metals in the industrial world. Large doses injected into closed cavities, as well as prolonged administration to burns (in the form of soluble bismuth compounds), can result in acute and perhaps fatal poisoning.
Environmental Effects of Bismuth
- Bismuth metal is non-toxic and provides little risk to the environment. Bismuth compounds are normally insoluble, however, they should be treated with caution because there is little knowledge of their effects and destiny in the environment.
Video Reference
References
- https://chemicalengineeringworld.com/bismuth-element-properties-and-information/
- https://www.rsc.org/periodic-table/element/83/bismuth
- https://chemistrytalk.org/bismuth-element/
- https://www.chemicool.com/elements/bismuth.html
- https://www.lenntech.com/periodic/elements/bi.htm
- https://www.radleys.com/blog/element-of-the-month-bismuth/
- https://www.chemistrylearner.com/bismuth.html
- https://pilgaardelements.com/Bismuth/Reactions.htm

