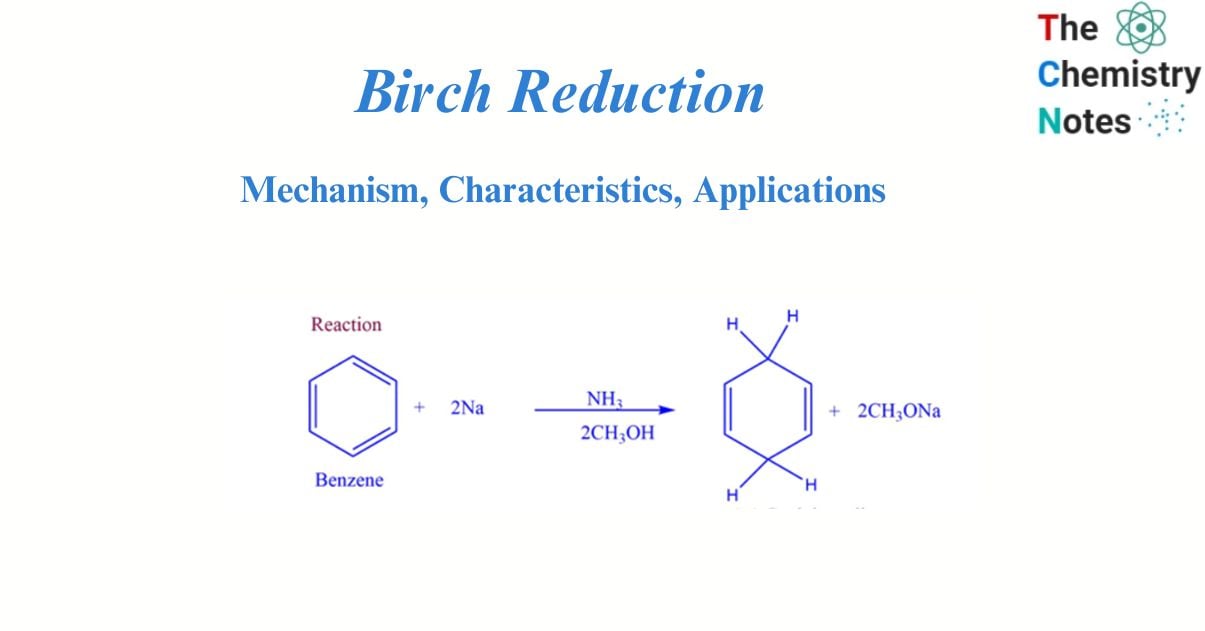
Birch reduction is a reduction reaction that converts aromatic compounds to dienes. The reaction is carried out in the presence of alcohol by sodium or potassium metal dissolved in liquid ammonia.
Australian scientist Arthur Birch initially published the Birch Reduction during World War II. When working at Oxford University’s Dyson Perrins Laboratory in 1937, Arthur Birch added to Wooster and Godfrey’s research. Arthur Birch was the first to describe the Birch reduction using sodium and ethanol. Lithium does increase yield. Birch reduction of aniline can also result in conjugated enamines. Birch Reduction can also be used to transform alkynes into alkenes.
What is Birch reduction reaction?
Birch reduction involves the reduction of aromatic substrate undergoes reduction with alkali metals in a mixture of ammonia and alcohol. In this reduction, diene undergoes 1.4-addition, and arenes give nonconjugated cyclohexadiene.
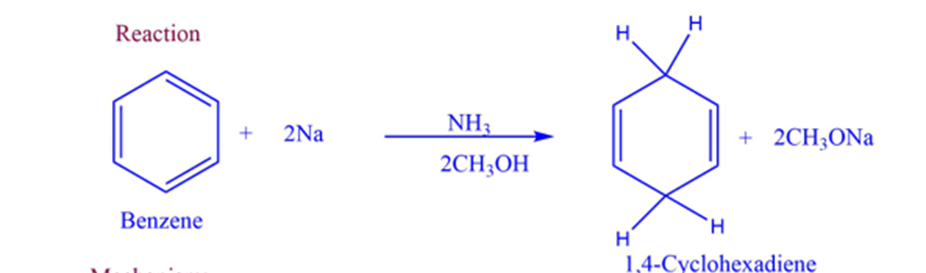
Mechanism of Birch reduction
- Step I: A radical anion is generated by a single-electron transfer from sodium metal to the aromatic ring.
- Step II: Proton transfer occurs from the solvent to the radical anion.
- Step III: The sodium metal transfers one electron to the radical.
- Step IV: The solvent’s proton is transferred to the anion created in the previous step.
Effect of substituents on birch reduction
When aromatic rings with electron-withdrawing or electron-donating groups are reduced by birch reaction, distinct compounds are produced in each condition.
Since the solvated electron in this reaction functions as a nucleophile, the presence of an electron-withdrawing group on the aromatic rings speeds up the process. However, the rate of reaction decreases when an electron-donating group is present on the rings because it reduces the positive character of the ring.
Characteristics of Birch reduction
- Alkali metals dissolve in liquid ammonia to produce blue liquid. Aromatic rings absorb electrons one at a time. When the initial electron is taken out, an ion is created.
- When an alcohol molecule loses its hydroxylic hydrogen, carbon-hydrogen bonds are created.
- When the second electron is taken out, cyclohexadienyl carbanion is created. The carbanion is inactive once the alcohol protonates it.
- Since this carbanion is cyclohexadienyl, it protonates at its center.
- It products with various structural compositions are produced by protonating the anionic radical at a particular site.
Applications of Birch reduction
- It transforms aromatic compounds with benzenoid rings into 1,4 cyclohexadiene.
- This is quite unlike catalytic hydrogenation, which typically reduces aromatic rings.
- It has the potential to be a very important and useful reaction. It is used to reduce aromatic and non-aromatic substances.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992
