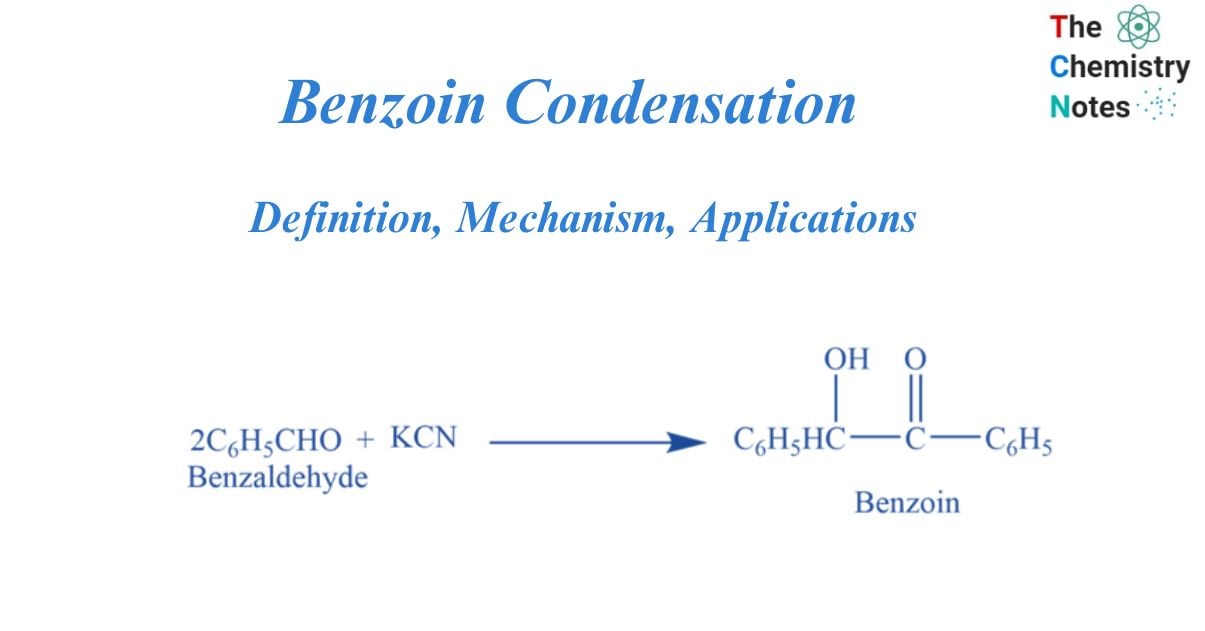
The Benzoin Condensation is a coupling reaction between two aldehydes that produces α-hydroxyketones. Only aromatic aldehydes could be converted using the initial procedures.
The condensation of two molecules of benzaldehyde to generate benzoin in the presence of a cyanide catalyst (e.g., NaCN and KCN) or thiamine (vitamin B) is known as the benzoin condensation. Benzoin has the structure of a ketone. It is made up of acetophenone with hydroxy and phenyl substituents at the alpha position (hydroxyl ketone). It has a molecular mass of 212.24 g/mol and is known by the IUPAC name 2-hydroxy-1,2-diphenylethanone.
Interesting Science Videos
What is a benzoin condensation reaction?
Two moles of benzaldehyde undergo a coupling reaction on warming with KCN to form benzoin. This is called a benzoin condensation reaction.

The formation of carbon-carbon bonds is a key process in benzoin condensation. It is accomplished by producing an acyl anion equivalent from one aldehyde molecule and adding it to another. Traditionally, a cyanide ion catalyzes the process.
Benzoin condensation is frequently catalyzed by a cyanide ion. Cyanide is utilized because it is an excellent leaving group, a good nucleophile, and can stabilize the intermediate ion. Condensation reactions are known as dehydration synthesis reactions because dehydration includes the loss of water, whereas synthesis involves the creation of new molecules.
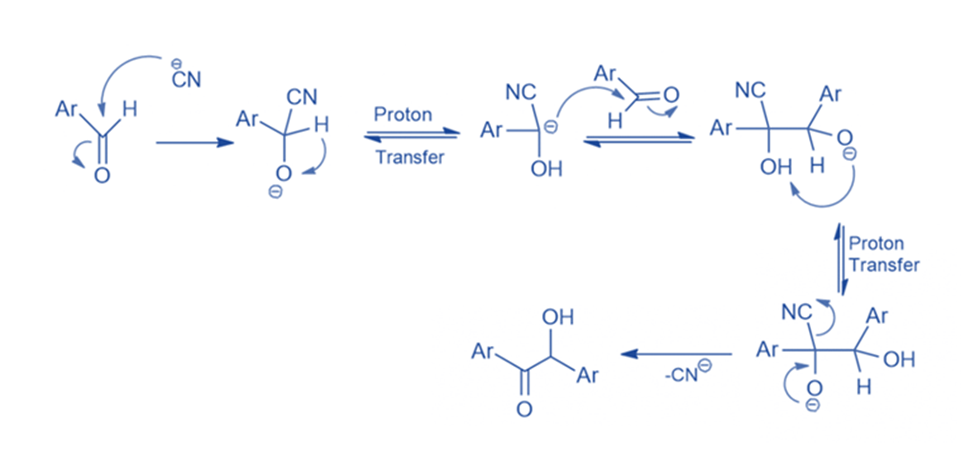
Mechanism of benzoin condensation
When aromatic aldehyde is treated with alcoholic KCN, the result is a hydroxy aromatic ketone known as benzoin rather than a cyanohydrin. The product of aromatic aldehydes with KCN differs from aliphatic aldehydes because, after CN- attack, the intermediate (I) in aromatic aldehydes has sufficient acidity (due to the -I effect of Ph) to allow intramolecular proton exchange to occur, resulting in a carbanion that is resonance stabilized. This carbanion then attacks another molecule of aromatic aldehyde, which undergoes intramolecular proton exchange and CN- CN ejection to yield the end product, benzoin. The attack of carbanion on the second molecule of aromatic aldehyde is the reaction’s rate-limiting phase.
Benzaldehyde is treated with a catalytic quantity of sodium cyanide in the presence of a base in the usual method for the benzoin condensation. The cyanide ion generates a stable intermediate cyanohydrin. Following this, the cyanohydrin combines with a second mole of benzaldehyde in a condensation process. The result is benzoin.
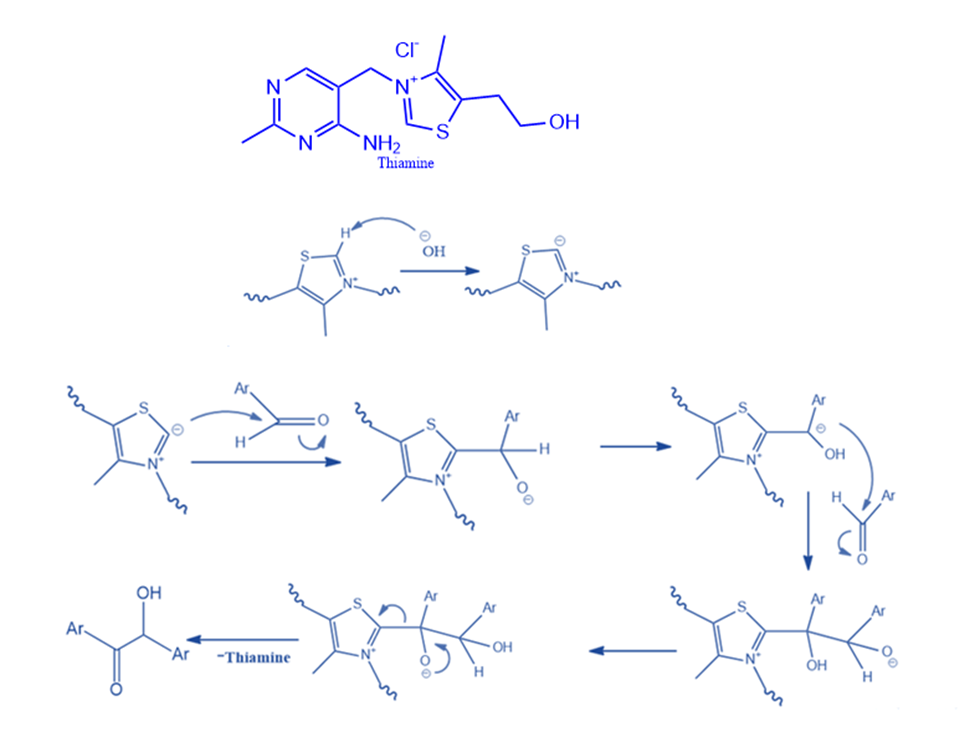
Thiamine-catalyzed benzoin condensation
The highly hazardous ion cyanide catalyzes various biological processes, including benzoin condensation. It was postulated that thiamine can behave similarly to cyanide in triggering these metabolic events as well as the benzoin condensation. To produce free thiamine, sodium hydroxide (NaOH) is added to thiamine hydrochloride. Thiamine (vitamin B1) is a colorless molecule with the chemical formula C12H17N4OS. The primary components of the reaction are the resonance-stabilized conjugate base of the thiazolium ion, thiamine, and the resonance-stabilized carbanion that it generates. The thiazolium ion, like the cyanide ion, possesses the ideal combination of nucleophilicity, the capacity to stabilize the intermediate anion, and favorable leaving group properties.
Application of benzoin condensation
- This process yields benzoins, which can be oxidized to benzyl or converted into a variety of diphenyl alpha-amino ketones or alcohols. This is one of the most common uses for benzoin condensation.
- Benzil can be produced by oxidizing benzoin with nitric acid. Benzil is used to cure polymer networks using free radicals.
- Benzoin is used in the hardening of various polymers by microemulsion.
- The reaction is useful in the synthesis of heterocyclic compounds as well as the aliphatic form of aldehydes.
- The reaction is also used in organic chemistry for the creation of polymers as well as the condensation of new monomers.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
