Benzoic acid is a simple aromatic carboxylic acid. The chemical formula is the C6H5COOH. It is an organic compound with a carboxyl group attached to a benzene ring and can be found in free form in gum benzoin, a natural resin derived from Java and Sumatra trees. It is a white crystalline solid that is widely used as a preservative and in the production of cosmetics, dyes, and plastics.
Interesting Science Videos
Laboratory preparation of Benzoic Acid
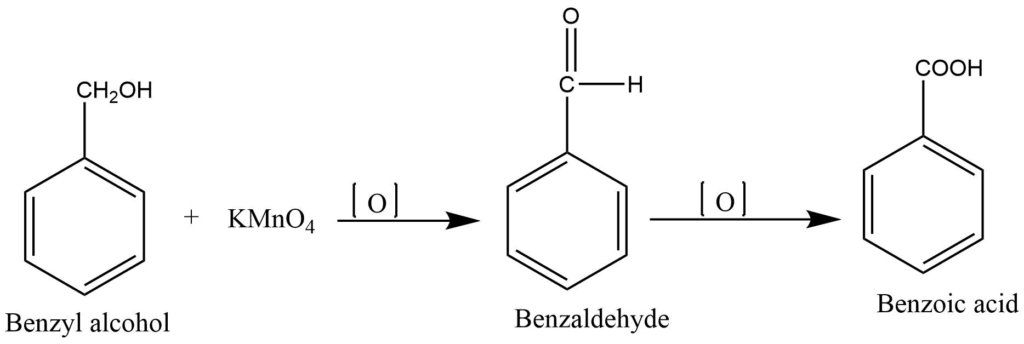
Oxidation of benzyl alcohol
The reaction of benzyl alcohol or benzaldehyde with alkaline KMNO4 gives benzoic acid.
Hydrolysis of phenyl cyanide with acid
Hydrolysis of phenyl cyanide with acid produces benzoic acid.
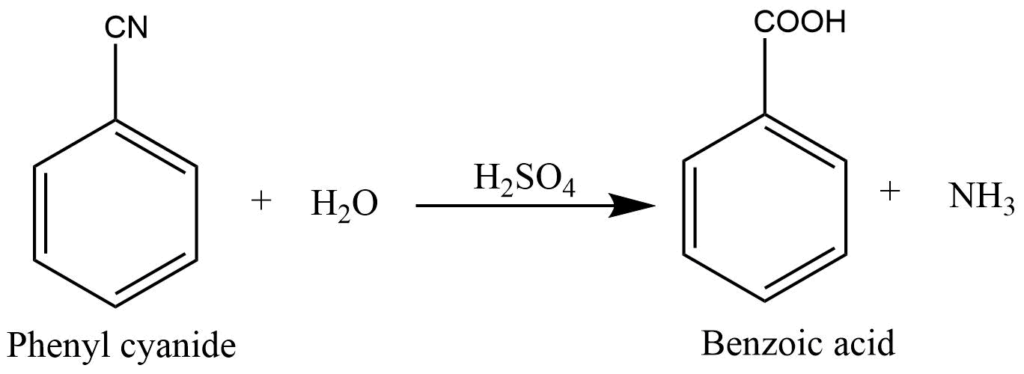
The action of carbon dioxide with Grignard reagent
Carbon dioxide reacts with the Grignard reagent to give the intermediate product. Which on hydrolysis produces carboxylic acid.
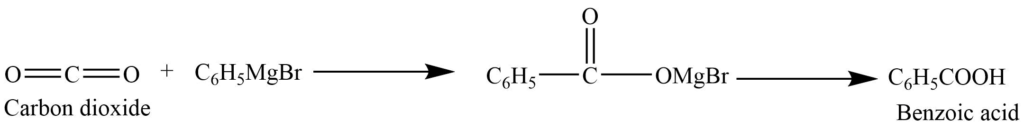
By Friedel – Craft’s reaction
Benzene reacts with the carbonyl chloride in presence of anhydrous aluminum chloride, benzoyl chloride is formed. Thus, formed benzoyl chloride on hydrolysis produces benzoic acid.
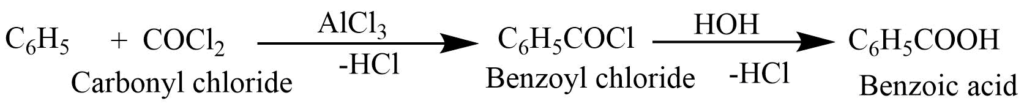
Commercial preparation of Benzoic Acid
1. Benzoic acid is produced by hydrolysis of benzene trichloride with calcium hydroxide in the presence of iron powder as a catalyst.
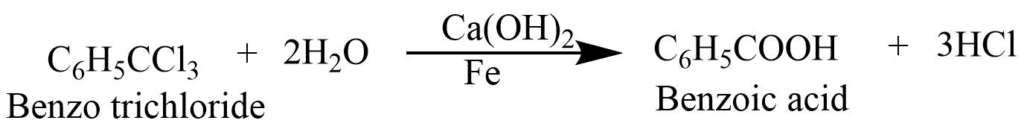
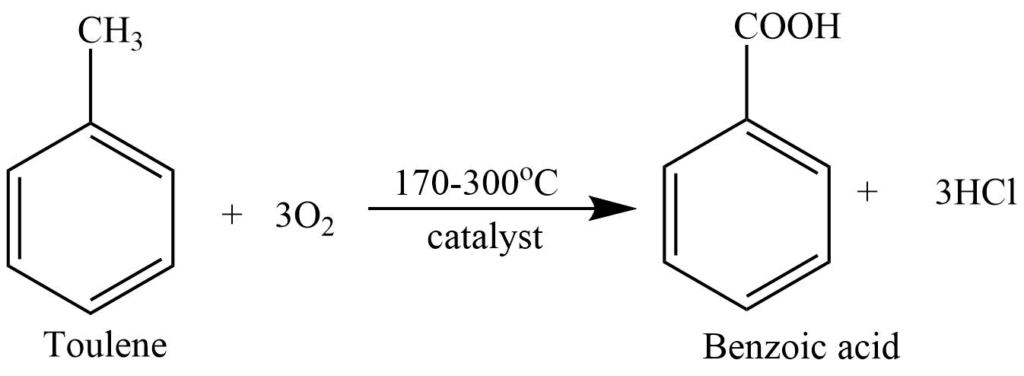
2. Toluene is catalytically oxidized with air and V2O5 or manganese and cobalt acetates to produce benzoic acid.
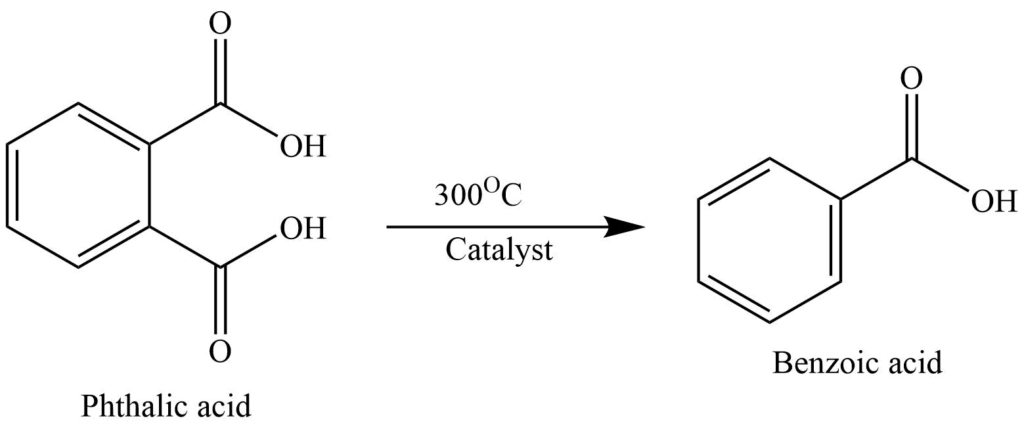
3. At 300oC, decarboxylation of phthalic acid in the presence of catalysts such as zinc, chromium, or nickel phthalate yields benzoic acid.
Physical properties of Benzoic Acid
- It is a white crystalline solid with a melting point of 122°C.
- It sublimes on quickly heating and is steam volatile
- Its vapor has an unusual pungent odor that causes sneezing and coughing.
- It is sparingly soluble in cold water but soluble in hot water, ether benzene, and alcohol.
Chemical properties of Benzoic Acid
It gives the reaction of both the carboxyl group and benzene nucleus.
The reaction of a carboxyl group
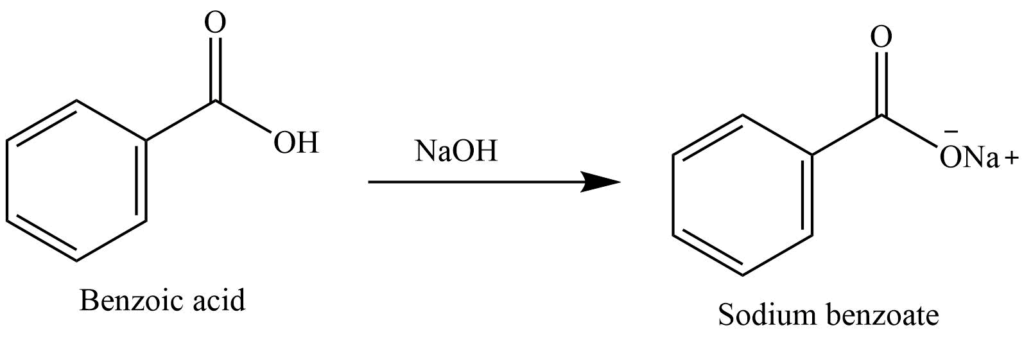
- On reaction with bases like sodium hydroxide, and sodium carbonate, it forms a salt.
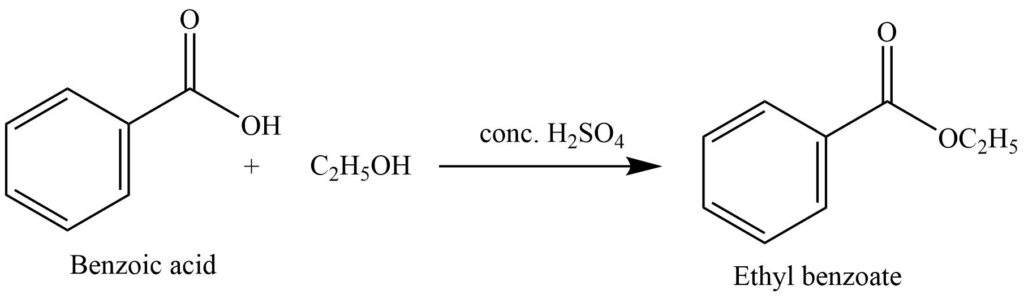
2. It reacts with the alcohol in presence of conc. sulphuric acid to form the ester. In this reaction, sulphuric acid acts as a dehydrating agent.
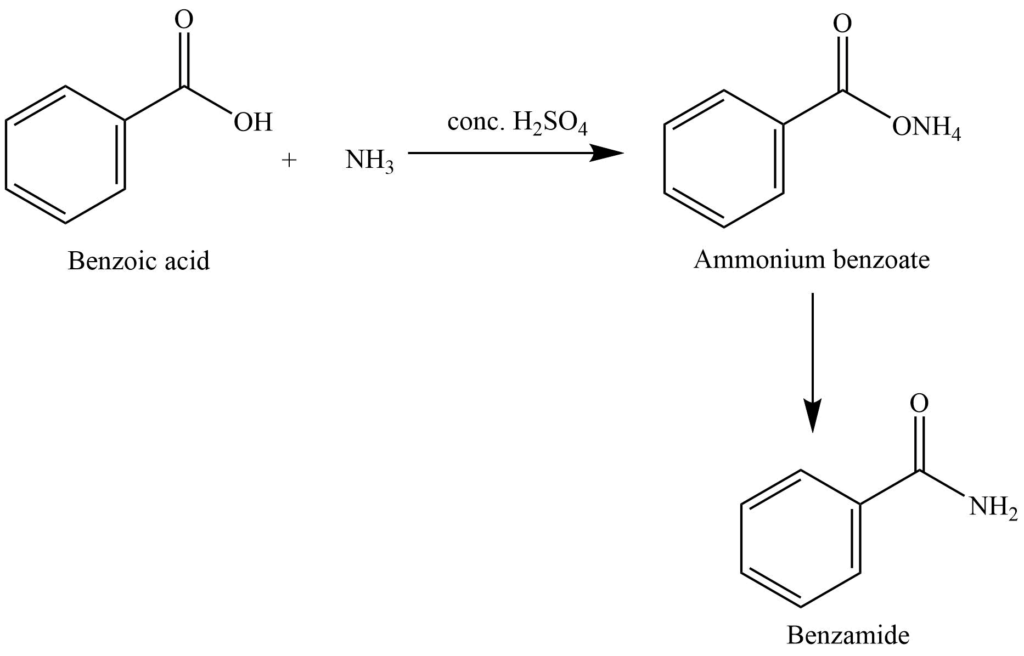
3. Reaction of benzoic acid with ammonia produces benzamide.
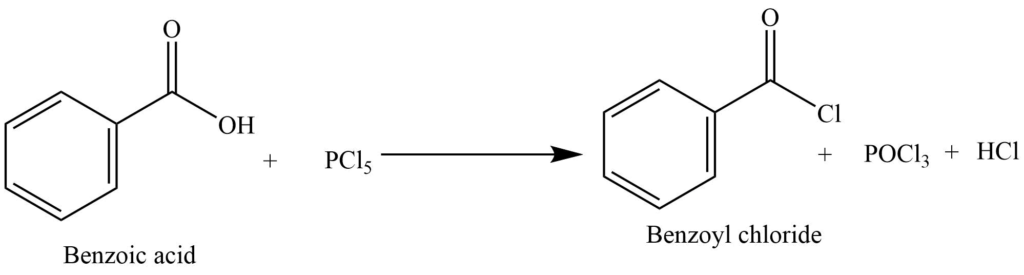
4. It reacts with phosphorous pentachloride to form benzoyl chloride.
5. Reduction with lithium aluminum hydride gives benzyl alcohol.
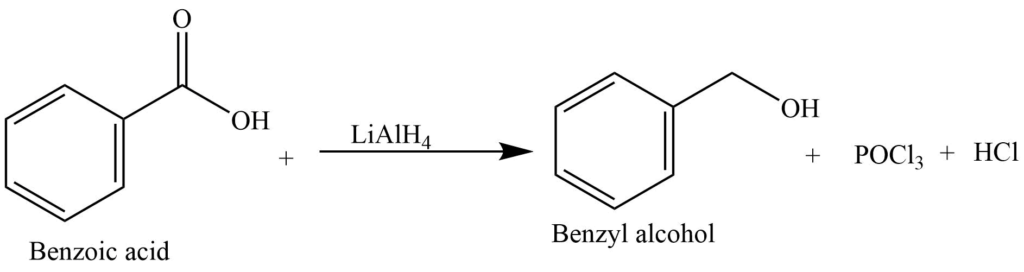
The reaction of a benzene nucleus
Benzoic acid shows a substitution reaction of the benzene nucleus.
1. Reaction of benzoic acid with conc. HNO3 produces m-nitro benzoic acid.
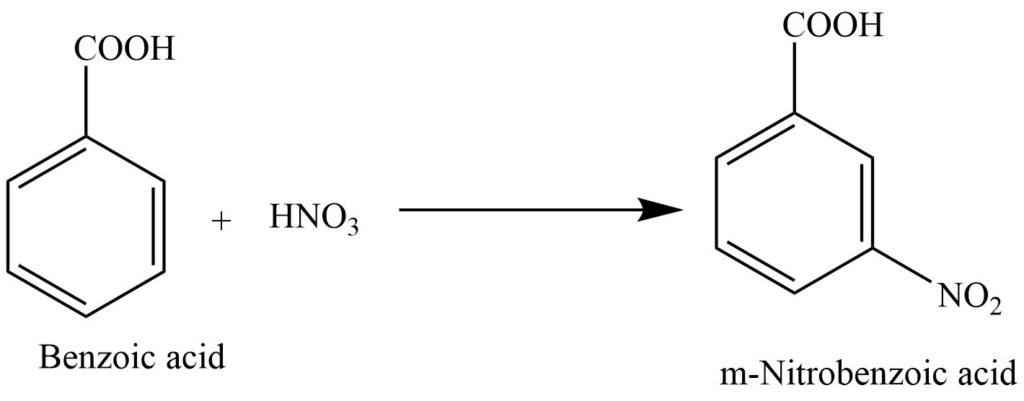
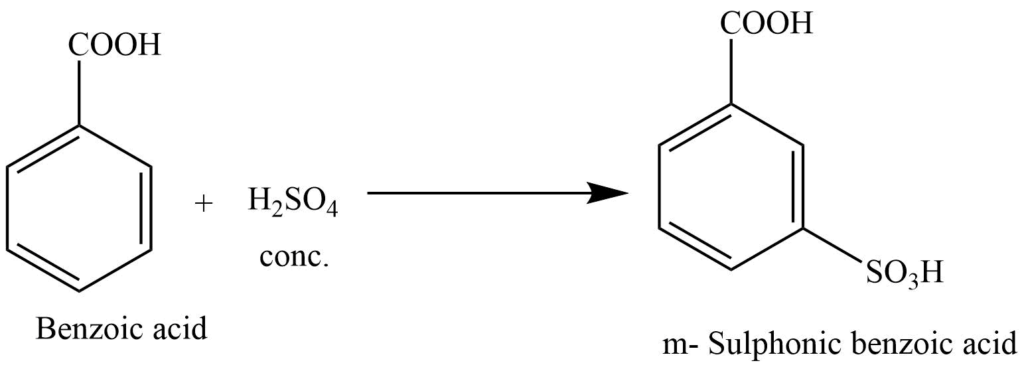
2. Reaction with conc H2SO4 produces m-sulphonic benzoic acid.
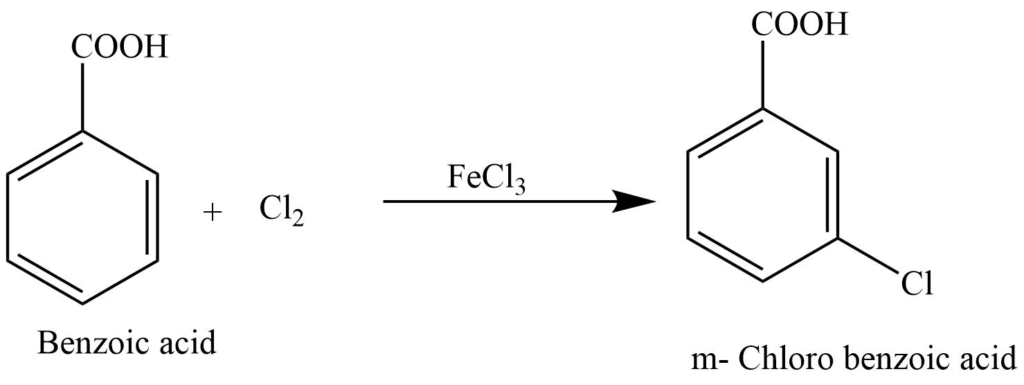
3.It reacts with chlorine in presence of FeCl3 to produce m-chloro benzoic acid.
Uses of Benzoic Acid
- It acts as the precursor for the synthesis of many organic compounds.
- Used in the manufacture of dyes, e.g., aniline blue
- It is used in an ointment for the treatment of many bacterial and fungal infections.
- Sodium benzoate is used as a food preservative.
- In vapor form, it is used as a disinfectant for bronchial tubes.
- It is a major component of facewash, mouthwash, and toothpaste
References
- https://www.shiksha.com/aldehydes-ketones-and-carboxylic-acids-preparation/uses-of-benzoic-acid-2725
- https://www.toppr.com/guides/chemistry/acids-bases-and-salts/benzoic-acid/
- https://www.vedantu.com/chemistry/benzoic-acid
- https://www.aakash.ac.in/important-concepts/chemistry/benzoic-acid
- https://byjus.com/chemistry/benzoic-acid/
- https://www.britannica.com/science/benzoic-acid
- https://www.drugs.com/mtm/benzoic-acid-and-salicylic-acid-topical.html
