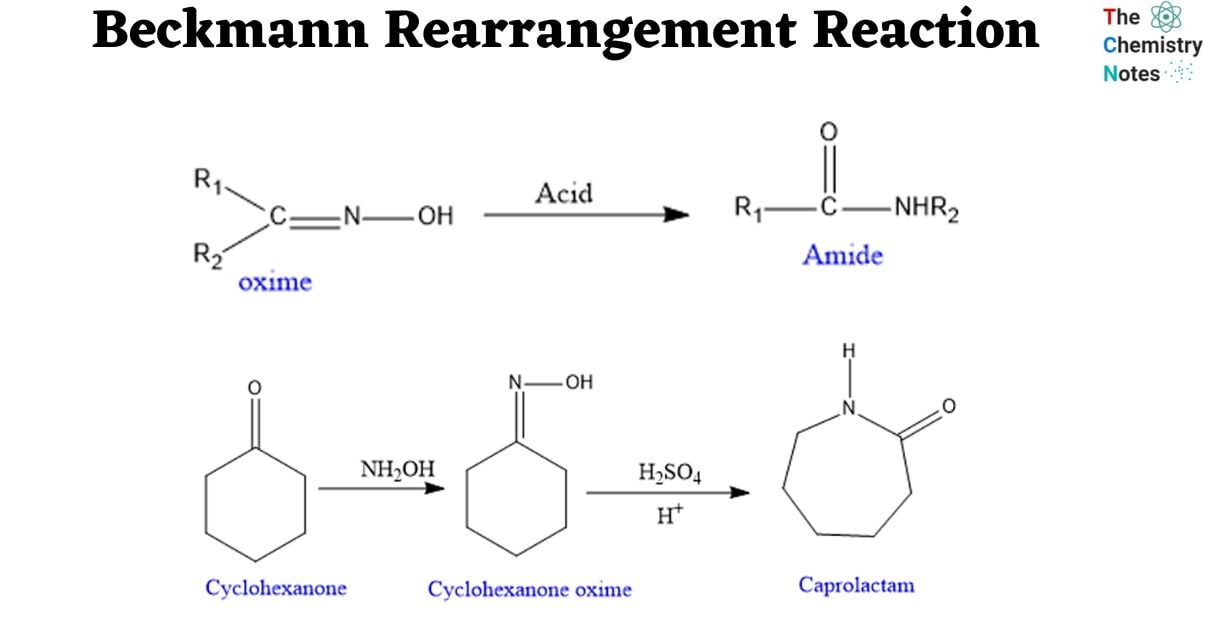
The Beckmann rearrangement reaction, named after the German scientist Ernst Otto Beckmann (1853–1923), is an acid-catalyzed conversion of an oxime to an amide. In the synthesis of pharmaceuticals and natural compounds, the Beckmann rearrangement is a sophisticated transformation that is often used.
What is Beckmann Rearrangement?
The Beckmann Rearrangement is an oxime rearrangement caused by acid that results in amides. Acid catalyst used are of wide and varied types, both protic and Lewis, for example: H2SO4, PCl5, SOCl2, RCOCl, BF3. Similar to the Hofmann, Schmidt, and Curtius Rearrangements, an alkyl migration is started in this reaction by the creation of an electropositive nitrogen.
Depending on the starting material, the Beckmann rearrangement of oximes can produce either amides or nitriles. Oximes made from aldehydes produce nitriles, whereas those made from ketones produce amides.
The process transforms oximes into their amides, allowing the nitrogen atom from the C=N bond to be introduced into the carbon chain and form a C-N bond. Depending on the starting material, it may also be capable to producing nitriles from aldehydes. Traditional techniques of rearrangement use severe reaction conditions, such as a strongly acidic medium and high temperatures, which can produce undesired byproducts and are inappropriate for sensitive substrates.
Beckmann Rearrangement Reaction Mechanism
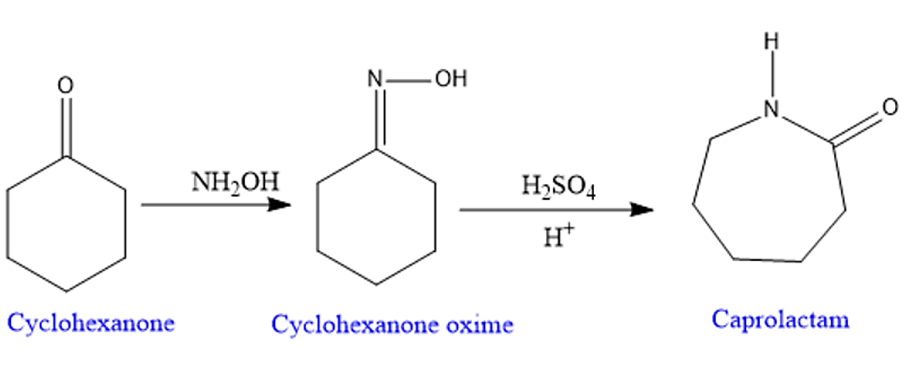
Step 1: Protonation- for the formation of good leaving group.
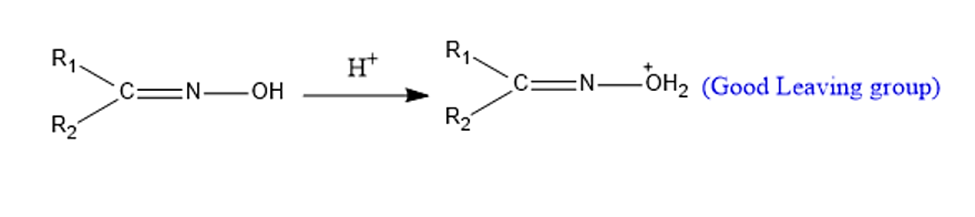
Step 2: Migration of Alkyl Group-To generate nitrilium cation, the alkyl group anti to the leaving group migrates followed by the removal of a water molecule.
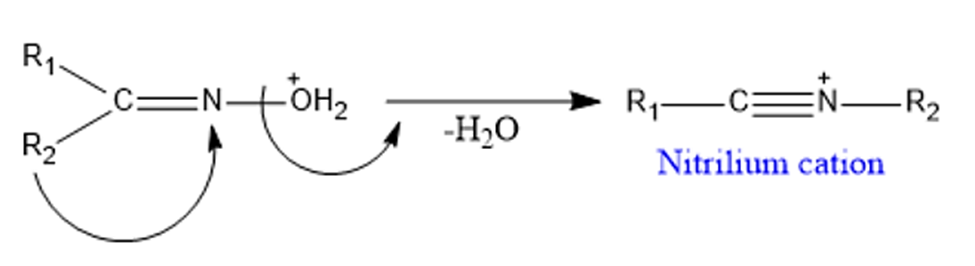
Step 3: Attack by water molecule to the nitrilium cation
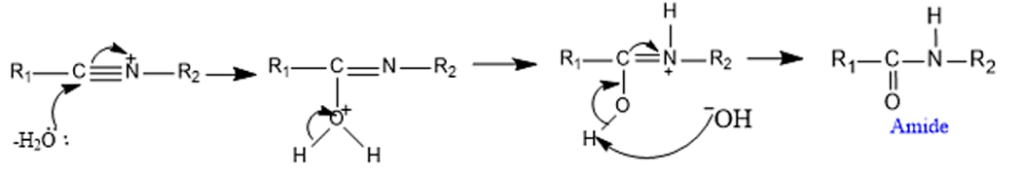
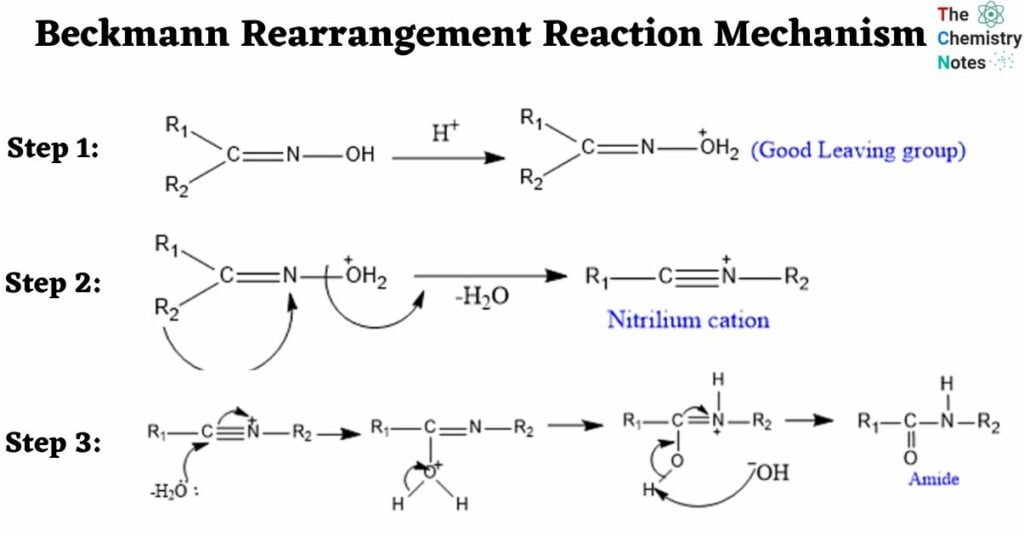
Application of Beckmann Rearrangement Reaction
- It is utilized in the synthesis of paracetamol in industry. This integration is accomplished by the use of hydroxylamine in the conversion of a ketone to a ketoxime.
- It is primarily utilized in the production of different steroids and medicines.
- Some chloro bicyclic lactams can be synthesized via the Beckmann Rearrangement.
- The Beckmann rearrangement is used to synthesize 17-progesterone, DHEA, benazepril, enprazepine, ceforanide, olanzapine, elantrine, prazepine, and etazepine.
- Hoechst-Celanese devised an industrial paracetamol synthesis that includes using a Beckmann rearrangement to convert a methyl ketone to an acetanilide.
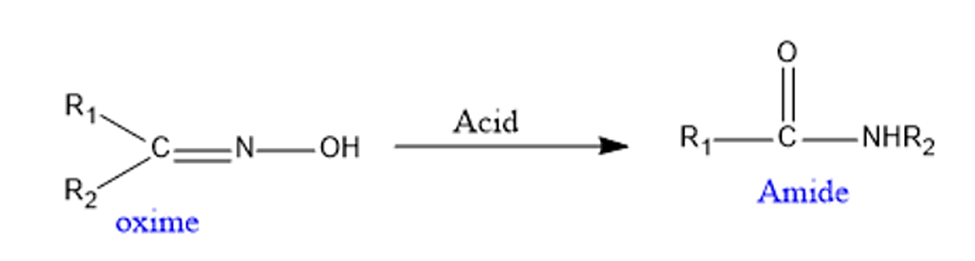
- It is used in the production of Nylon 6 raw material. Nylon – 6 is derived from caprolactam, a basic material. Caprolactam can be produced through the Beckmann rearrangement reaction of cyclohexanone and oxime.
Video on Beckmann Rearrangement Reaction
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- JONES, B. Mechanism of the Beckmann Rearrangement. Nature 157, 519 (1946). https://doi.org/10.1038/157519a0
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- https://testbook.com/chemistry/beckmann-rearrangement

