Atomic structure is the arrangement of subatomic particles within the atom. An atom is a complicated configuration of negatively charged electrons grouped around a positively charged nucleus in certain shells. Protons and neutrons make up the majority of the atom’s mass, except hydrogen, which has just one proton. An atom has a diameter of roughly (2-3) Å. Every atom is about the same size. The angstrom (Å), which is defined as 1×10-10 meters, is a useful unit of length for calculating atomic sizes.
John Dalton was the one who initially explained the law on multiple proportions back in the year 1800. An atom stands for the basic building blocks that can be broken down into subatomic particles. Atomic models are created as a result.
Interesting Science Videos
Discovery of Electrons, Protons, And Neutrons
Discovery of Electrons
In the year 1897, J. J. Thomson discovered the electron, which established the beginning of modern atomic physics. The electrons, which are negatively charged, move at random inside predetermined energy shells encircling the nucleus. The number and configuration of an atom’s electrons determine most of its characteristics. An electron has a mass of 9.1 x 10-31 kg.
Discovery of Protons
The nucleus contains two distinct kinds of particles: neutrons and protons. The proton is one of these neutrons. In 1919, Sir Ernest Rutherford presented evidence that the nucleus contains a positively charged particle called a proton. This discovery was monumental at the time. The positive charge of the electron is equal to but opposite to that of the proton. The number of protons an atom has in its nucleus identifies the type of chemical element to which it belongs. The mass of a proton is approximately 1.67 x 10–27 kg.
Discovery of Neutrons
Neutrons are the other particle that can be discovered in the nucleus. A scientist from the United Kingdom named Sir James Chadwick was the one who made the discovery. The neutron shares the mass of the proton and does not possess an electrical charge of its own. The electron cloud and the nucleus do not repel the neutron because it lacks an electrical charge. Because of this, the neutron is an effective tool for studying the structure of the atom.
Structure Of Atoms
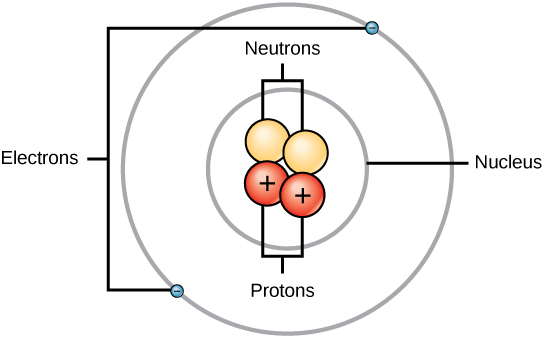
Each atom is composed of a nucleus and electrons that are bonded to that nucleus. The term “nucleon” refers to both protons and neutrons that are present in the nucleus. The mass of an atom is almost entirely determined by its nucleus, making it the atom’s most important component. The electron, the proton, and the neutron are the fundamental building blocks of the atomic structure. The behavior of an atom is directly related to the orbital in which its electrons are circling, and the shells are responsible for determining the chemical properties of an element.
Subatomic Particles
Protons
Protons are positively charged subatomic particles. The charge of a proton is 1e, which amounts to roughly 1.602 × 10-19.
The mass of a proton is roughly 1.672 × 10-24.
Protons are almost 1800 times heavier than electrons.
The total number of protons in the atoms of an element is always equal to its atomic number.
Neutrons
The mass of a neutron is roughly the same as that of a proton, i.e., 1.674×10-24.
Neutrons are electrically neutral particles and carry no charge.
Different isotopes of an element have the same number of protons but differ in the amount of neutrons present in their individual nuclei.
Electrons
The charge of an electron is -1e, which approximates to -1.602 × 10-19.
The mass of an electron is roughly 9.1 × 10-31.
Due to the comparatively low mass of electrons, they are omitted when determining the mass of an atom.
The General Structure Of Atoms
Protons and neutrons have positive charges, while electrons have negative charges. Atoms become neutral when their electron and proton distributions are balanced; otherwise, they ionize. Depending on the number of electrons and protons an atom may have, it can be evaluated as having a charge that is positive or negative.
- Electromagnetic force binds electrons and nuclei.
- Protons and neutrons employ nuclear force to attract each other inside the nucleus.
- The presence of multiple protons shows the atomic number.
- Similarly to that, numerous neutrons describe the isotopes.
- The magnetic characteristics of the element can be attributed to the presence of electrons in it.
- Atomic interaction and distraction from each other contribute to physical changes in the surroundings. This can be done through chemical bonding between them.
Properties of Atom
The properties of an atom can be described depending on the number of protons, neutrons, and electrons. Here we are going to look at the properties of an atom.
Atomic Number
The atomic number, which corresponds to an atom’s chemical properties, is determined by the number of protons present in the nucleus. Z represents the standard symbol for an atomic number. There is no doubt that positively charged particles have an impact on atomic number. When protons and electrons are precisely balanced among the elements, a neutral atom is produced. The stable atoms include carbon.
Atomic Mass
The nucleus of an atom is what primarily determines its weight or mass. The sum of the neutrons and proton masses determines the weight of the nucleus. Atomic mass is denoted by the letter A. The term “isotopes” refers to a substance whose neutron and proton counts differ.
Radioactive
Changes in a nucleus are caused by fluctuations in the number of protons and neutrons that make up the nucleus. When this occurs, the associated atom is referred to as “radioactive. This state is nothing more than a nucleus that is unstable. The atoms will remain in the same state for the duration of this stage until the system becomes stable. Radioactivity is a property of elements that are defined by an atomic number that is higher than the threshold of 82.
The Spin of Electrons
The spinning of electrons has a characteristic that falls within fermions: it only requires half of the integer spin. Helium, for instance, is an element that has an atomic number of 2 based on the number of protons it contains. It should be spun in different directions for it to occupy the same orbit. They are regarded as small magnets because of the movement of an electric charge as a result. Indeed, the magnetic moment of an electron is about 9.28 × 10–24 joules per tesla.
Electric Charge
Ions are formed when an atom is involved in the motion of an electron and the formation of bonds for the purpose of achieving stability. This ends up resulting in the development of an ion. During this transformation, the status of an atom changes from positive to negative depending on whether or not it is the donor or the receiver.
Relative Atomic Mass
The relativity in atomic mass is average when compared to a single carbon atom because the number of protons in carbon is equal to the number of neutrons, which makes carbon an element with neutral atomic status.
Atomic Isotopes
A significant quality of an atom is its atomic number, which can be understood in terms of the number of protons within it. An atom’s chemical characteristics are dictated by its atomic number, which is represented by the symbol Z. The atomic mass number is equal to the sum of an atom’s total number of nucleons (which includes both protons and neutrons). This value is represented by the letter A. The symbol N is used to signify the number of neutrons that are present in an atom. Therefore, the formula for determining an atom’s mass is A = N + Z.
Isotopes are atoms that have the same atomic number but different atomic masses. Isotopes have chemical properties that are identical but have drastically distinct nuclear properties. For example, hydrogen has three isotopes. Two isotopes are radioactive, while tritium (one proton and two neutrons) is unstable. The majority of elements have stable isotopes. Elements can also produce radioactive isotopes.
Atomic Structures of Some Elements
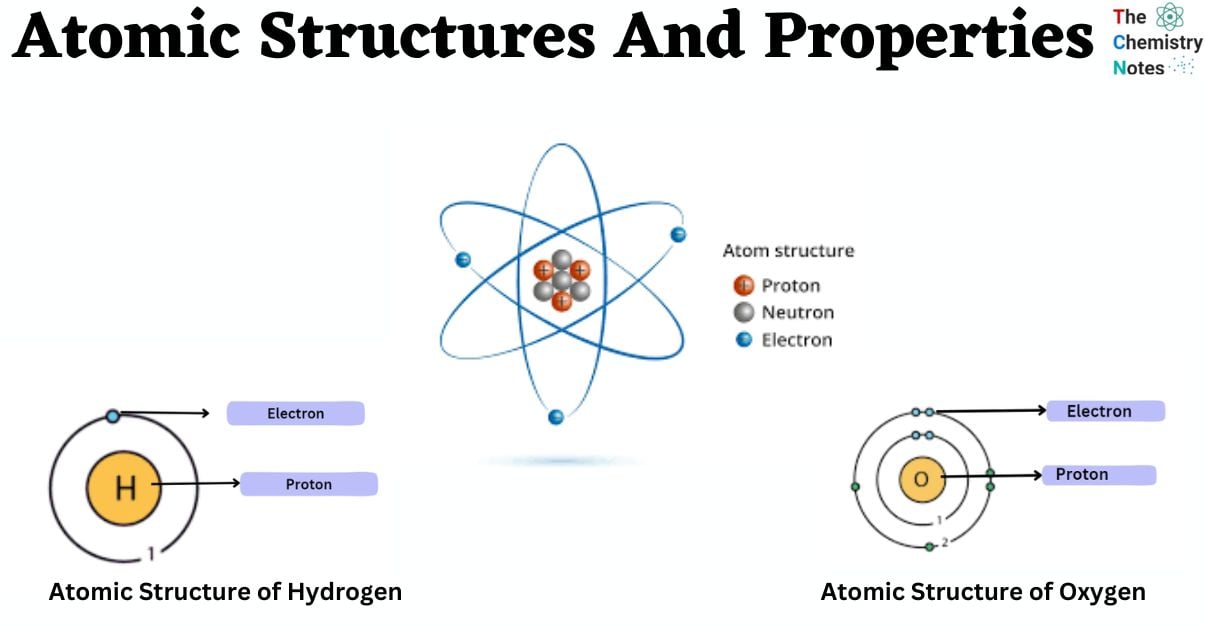
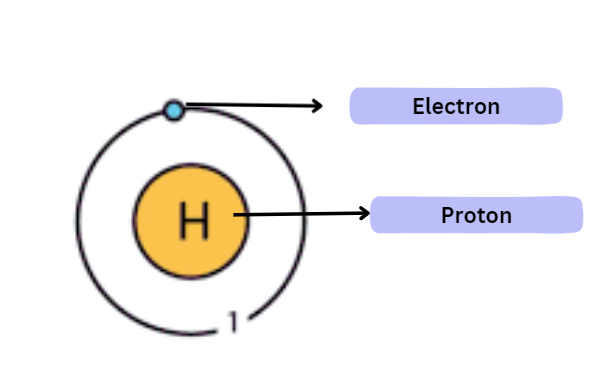
Hydrogen
Protium is the most prevalent hydrogen isotope on Earth. This isotope has an atomic number of 1 and an atomic mass number of 1.
This indicates that the hydrogen atom has a single proton, one electron, and no neutrons (total number of neutrons = mass number – atomic number).
Carbon
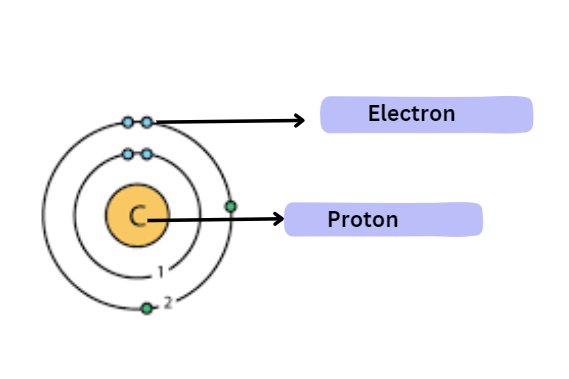
12C and 13C are the two stable isotopes of carbon. The natural abundance of 12C is 98.9% among these isotopes. There are 6 neutrons, 6 electrons, and 6 protons in it.
Atomic structure of carbon: The electrons are distributed among two shells, with the valence shell having the most electrons at four. Carbon can establish a number of chemical connections with different elements thanks to its tetravalency.
Oxygen
There are three stable isotopes of oxygen, and they are referred to as 18O, 17O, and 16O. However, the oxygen isotope with the highest abundance is 16O.
The structure of the atom of oxygen: This isotope has 8 protons and 8 neutrons since its atomic number is 8 and its mass number is 16. In an oxygen atom, the valence shell is home to 6 of the atom’s 8 electrons.
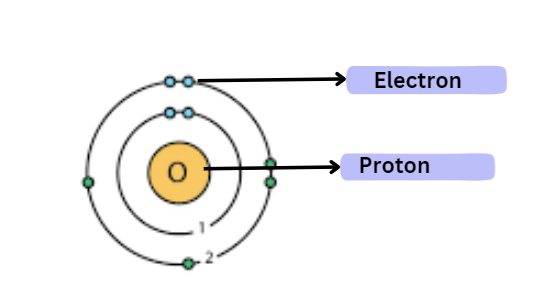
Atomic Models
Several atomic models were proposed and rejected before the correct one was eventually discovered. let us explore the various atomic models that were proposed and ultimately rejected before the final model was accepted as the most widely recognized.
Dalton’s model of the atom
- Dalton proposed a well-known theory that states that matter is made up of tiny particles called atoms.
- Atoms are considered indivisible, meaning they cannot be divided, and they cannot be destroyed through chemical reactions.
- All atoms belonging to a particular element exhibit the same chemical characteristics and possess the same mass.
Limitations
- One of the drawbacks of Dalton’s theory is that he stated that atoms of the same element are identical in all aspects, while atoms of different elements are distinct in all aspects.
- It was discovered by several scientists that atoms consist of electrons, protons, and neutrons.
J.J. Thomson’s model of the atom
- Thomson proposed a theory in which he defined atoms as being similar to a Christmas pudding.
- He explained that atoms are composed of a positively charged sphere with electrons embedded within it.
- In addition, he stated that the negative and positive charges possess equal magnitudes, resulting in an atom being electrically neutral as a whole.
Limitations
- One of the main limitations is that it does not account for the existence of the nucleus, which was later discovered by Ernest Rutherford.
- Additionally, Thomson’s model does not explain the distribution of electrons within the atom or their energy levels. Finally, the model does not account for the phenomenon of spectral lines, which are observed when atoms emit or absorb light.
- It did not provide an explanation for the stability of an atom.
Rutherford’s Atomic Model
- Rutherford’s atomic model is a significant contribution to the field of atomic physics.
- Rutherford conducted an experiment in which he utilized alpha particles to scatter off of a sheet of gold.
- He observed that a majority of the α-particles passed through the gold foil without any deflection.
- Some alpha particles were deflected at small angles, while others were deflected at nearly 180 degrees.
- Rutherford concluded from this experiment that atoms have a nucleus, which is a positively charged spherical center. He also found that almost all of the atom’s mass is concentrated in the nucleus, which has a radius of 10^-15 meters, while the atom itself has a radius of 10^-10 meters.
- According to the theory, the nucleus is significantly smaller than the atom in size.
- He stated that electrons orbit the nucleus in a well-defined path known as a “orbit.”
Limitations
Based on our experiment, we have determined that J.J. Thomson’s proposed model cannot be accurate. This is because his model suggests that the atom is not hollow, which our findings have contradicted. The atom’s positive charge is evenly distributed throughout its volume. If this statement is accurate, then it follows that a significant number of alpha particles would be scattered due to the repulsive force generated by the positively charged nucleus and the alpha particles.
- However, we obtain the exact opposite outcome. The majority of alpha particles pass through gold foil without deviation. This observation led to the understanding that the positive charge of an atom is concentrated in a small, dense nucleus at its center. This finding supported Rutherford’s Nuclear model hypothesis.
- According to the atomic model, the positive charge within an atom is concentrated at its center, while the electrons orbit around it in a manner similar to planets orbiting the sun.
- Due to certain issues, such as the instability of the nucleus, the Bohr model was eventually adopted.
Neil Bohr’s theory
- In his theory, Bohr employed the concept of quantization and presented the following ideas.
- Electrons orbit without losing energy.
- Electrons transition from lower to higher energy states by absorbing energy, and transition from higher to lower energy states by releasing energy.
- The ground state is the term used to refer to the lowest energy level of an electron.
- The Quantum number is an integer that represents the energy levels. The quantum numbers begin with n=1, 2, 3, and so on, and are designated as K, L, M, N, and so forth. Shells.
Limitations
- Bohr’s model did not account for the impact of magnetic and electric fields on atomic spectra.
- The Bohr atomic model accurately predicted the behavior of smaller atoms such as hydrogen. However, when larger atoms were taken into consideration, the model produced inadequate spectral predictions.
Frequently Asked Questions (FAQ)
How can you describe Atomic structure?
Atoms are made up of a nucleus that is comprised of positively charged protons and neutrons, as well as shells of electrons that orbit the nucleus.
What are subatomic particles?
Subatomic particles are the fundamental particles that make up an atom. This term generally refers to the particles known as protons, electrons, and neutrons.
What is an atom?
Atoms are defined as the fundamental unit of matter that possesses all the characteristic properties of a specific element while being the smallest possible size.
Molecules are formed through the process of atoms coming together and collaborating.
Video on Atomic structure
References
- https://byjus.com/jee/atomic-structure/
- https://www.geeksforgeeks.org/atomic-structure/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/atomic-structure/
- https://unacademy.com/content/railway-exam/study-material/chemistry/atomic-structure-and-properties/
- https://www.varsitytutors.com/ap_chemistry-help/atomic-structure-and-properties?page=4
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura.General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall, 2007.
- https://testbook.com/chemistry/different-atomic-models

