Atomic absorption spectroscopy determines the concentration of elements in a liquid sample by absorbing energy from specific wavelengths of light (often 190 to 900 nm). It is a technique for determining the concentration of metal atoms/ions in a material. Atomic absorption spectrometry (AAS) detects elements in liquid or solid samples by using certain wavelengths of electromagnetic radiation from a light source. Individual elements absorb wavelengths differently, and the absorbances of these elements are evaluated against standards.
Atomic absorption spectroscopy has become one of the most commonly utilized analytical chemistry methods. This is because the technique provides appropriate sensitivity for many applications and is mainly interference free for determining most metals and metalloids.
Interesting Science Videos
History of atomic absorption spectroscopy
Atomic absorption spectrometry was discovered as a phenomenon in 1802 when the English scientist William Hyde Wollaston noticed and reported dark lines in the sun’s spectrum. In 1817, the German physicist Josef von Fraunhofer carefully plotted out these spectral absorption lines, which are today named after him. In 1860, the physicists Gustav Kirchhoff and Robert Bunsen established a theory of spectrochemical analysis. Kirchhoff and Bunsen invented the spectroscope, which splits light into wavelengths. This approach did not become widely employed until the 1930s.
However, atomic absorption spectroscopy as a modern technology for chemical research dates from 1955, when Alan Walsh, a Lancashire-born physicist, published an important paper on the prospects for atomic absorption spectroscopy in Melbourne, Australia. Walsh’s breakthrough came when he realized he needed to measure light absorption rather than emission. This resulted in the development of new atomic absorption spectroscopy procedures. In the 1960s, the first commercially available instruments arrived.
With the continued application of new technologies, such as automation and computers, Atomic absorption spectrometry was evolved into an exceptionally reliable analytical procedure. It is quick, sensitive, specific, and easy to use.
Principle of atomic absorption spectroscopy
The basis of atomic absorption spectroscopy (AAS) is that free atoms in their ground state can absorb light of a specific wavelength.
When a metallic salt (or any metallic compound) solution is put to a flame, a vapor containing atoms of the metal may develop. Most of these gaseous metal atoms will stay in their ground state. These ground-state atoms can absorb radiant light of their unique resonance wavelength. As a result, if the light of resonance wavelength passes through a flame containing the atoms in question, some of the light will be absorbed, and the extent of absorption will be proportional to the number of ground state atoms present in the flame.
This is the underlying principle of Atomic absorption spectrometry. In Atomic absorption spectrometry as with molecular absorption, the absorbance (A) is given by the logarithmic ratio of the intensity of the incident light signal (I0) to the intensity of the transmitted light (It).
A = log ( Io / I)
= K L NO
where,
No = the concentration of atoms in the flame (number of atoms per mL)
L = the path length through the flame
K = a constant related to the absorption coefficient
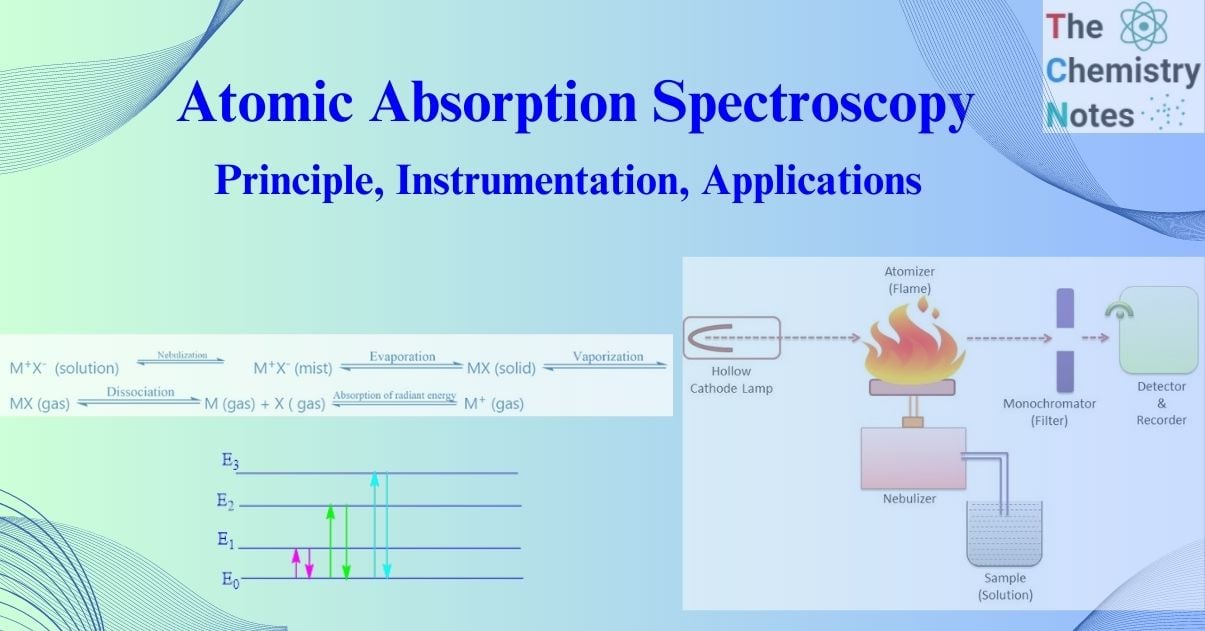
The procedure by which gaseous metal atoms are produced in the flame may be
represented as follows:
M+X– (solution)  M+X– (mist)
M+X– (mist) 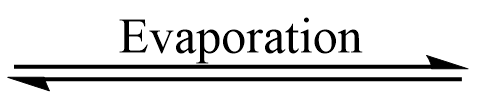 MX (solid)
MX (solid) 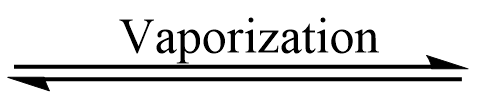 MX (gas)
MX (gas) 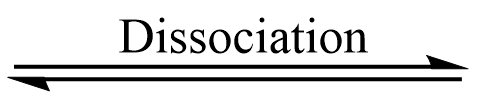 M (gas) + X ( gas)
M (gas) + X ( gas)  M+ (gas)
M+ (gas)
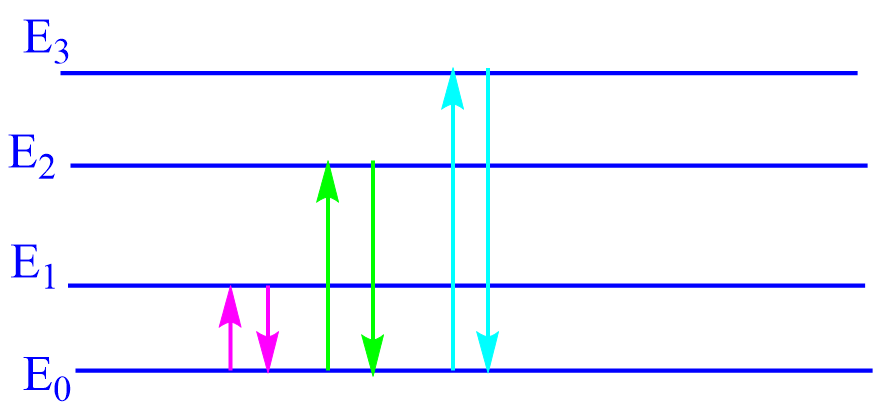
In the above-simplified energy level diagram of atoms, Eo represents the ground state in which
the electrons of a given atom are at their lowest energy level and E1, E2, E3, etc. represent excited energy levels. The transition between two quantized energy levels, say from Eo to E1, E2 corresponds to the absorption of radiant energy, and the amount of energy absorbed (∆E)
is determined by Bohr’s equation.
∆E = E1 – Eo = hv = h C / λ
Where,
C = Velocity of light
h = Planck’s constant
v = frequency of the radiation absorbed
λ = wavelength of the radiation absorbed.
Most of the atoms in a typical flame are in the ground electronic state rather than the excited state. As the ratio of excited to ground state atoms is very small, the absorption spectrum of a given element is only associated with transitions from the ground state to higher energy levels. So, it is thus easier to observe than the emission spectrum.
The relationship between the ground state and the excited state population is given by the
Boltzmann equation.
(N1 / No) = (g1 / g0) e-∆E / KT
N1 = number of atoms in an excited state
No = number of ground state atoms
(g1 / g0) = ratio of statistical weights for ground and excited states
∆E = energy of excitation = hv
K = the Boltzmann constant
T = the temperature in Kelvin
(N1 / No) depends upon both ∆E and T. An increase in T and a decrease in AE will result in a higher value for the ratio calculation shows that only a small fraction of the atoms are excited, even under the most favorable conditions i.e. when T is high and ∆E low.
Instrumentation of atomic absorption spectroscopy
For all types of atomic absorption spectrometers, the following components are required
I. Source of radiation eg. Hollow cathode lamp
II. Chopper
III. Nebulizer-burner system (Atomization unit)
IV. Atomizer
V. Monochromators
VI. Detectors (Photometric measuring system)
VII. Amplifiers
VIII. Read out device (Recorder)
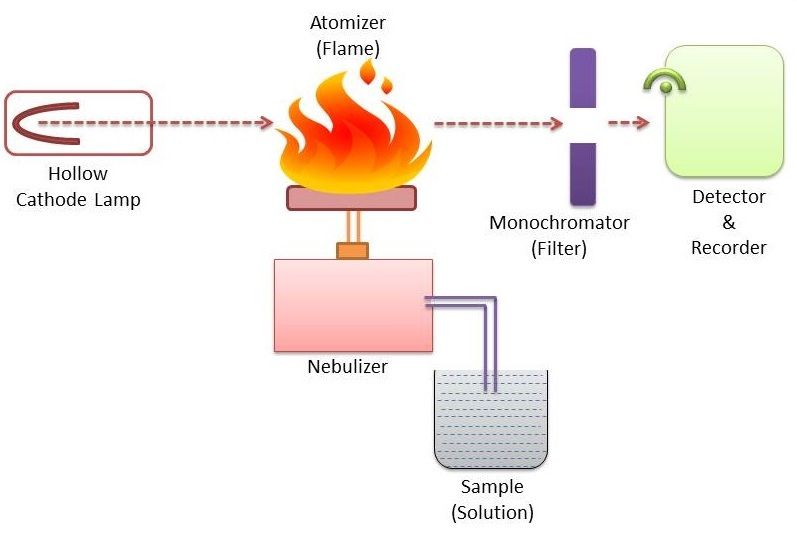
Instrumentation of atomic absorption spectroscopy
Image source: https://www.sciencedoze.com/2022/09/atomic-absorption-spectroscopy.html
I. Light source (Source of radiation )
The primary need of the light source is to generate a narrow line profile with a minimum background. It should also have a reliable and reproducible output with enough intensity to provide a high signal-to-noise ratio. In, atomic absorption spectroscopy generally, two types of light sources are used i.e. hollow-cathode lamps (HCL) and electrodeless discharge lamps (EDL).
Except for analytes such as arsenic and selenium, hollow-cathode lamps are the most typically employed, while electrodeless discharge lamps are used less frequently than HCLs.
II. Chopper
The light source should be modulated and mechanically chopped to differentiate between the light from the source and the emission from the sample cell. Chopper distinguishes between absorption and emission signals. It is situated between the light source and the atomizer.
III. Nebulizer – burner system
It produces a mist or aerosol of the test solution i.e. it converts the test solution to gaseous atoms It sucks in liquid samples at a controlled rate and produces a fine aerosol spray for use in the flame. It effectively combines aerosol, fuel, and oxidant.
IV. Atomizer
Atomization is the process of separating particles into individual molecules and breaking molecules down into atoms. This is done by heating the analyte in a flame or graphite furnace to high temperatures.
To generate atoms from a sample, two systems are usually utilized. Aspiration involves sucking a sample solution into a flame, whereas electrothermal atomization involves placing a drop of sample into a graphite tube that is subsequently heated electrically. Some instruments include both atomization processes but only have one pair of lights. After selecting the suitable lamp, it is directed toward one or more atomization systems.
V. Monochromator (Prisms Gratings)
A line isolation device is necessary to ensure that only light of a wavelength specific to the analyte of interest is measured. A monochromator is the line isolation device utilized for atomic absorption. All wavelengths of light enter the monochromator through an entrance slit and are subsequently divided into specific wavelengths using a prism or, more typically, a diffraction grating.
It isolates a specific emission line from other lines. It separates the resonance line from all non-absorbed lines generated by the radiation source. By adjusting the position of this dispersing device, only light of the desired wavelength enters the detector through the exit slit. Since atomic absorption is a specialized technique, very highly resolving monochromators are not required.
VI. Detector
The monochromator’s selected light is directed onto a detector. This apparatus converts radiant energy into electrical energy, giving an electrical signal to the radiant absorbed by atoms. Typically a photomultiplier tube is used as a detector. Its function is to convert the light signal into an electrical signal proportional to the light intensity.
VII. A-C amplifier
The electric current from the photomultiplier detector is fed to the amplifier which
amplifies the electric current several times.
VIII. Read-out devices
It may be a simple galvanometer or chart recorder. digital display etc.
Summary working procedure of atomic absorption spectroscopy
Light of a specific wavelength (emitted by a special type of lamp) that is capable of emitting the spectral lines corresponding to the energy required for an electronic transition from a ground state to an excited state is permitted to pass through the flame. At that time, the sample solution is drawn into the flame. Before entering the flame, the solution is dispersed into a mist of very small droplets, which evaporates in the flame to produce first the dry salt, and subsequently the salt vapor. A portion of this vapor will dissociate into atoms of the elements to be investigated.
As a result, the flame contains free unexcited atoms capable of absorbing radiation from an external source when the radiation matches exactly the energy required to transition the element from the ground electronic state to the higher excited state.
The unabsorbed radiation from the flame is then allowed to travel through a monochromator, which isolates the exciting spectral lines of the light source. The unabsorbed radiation from the monochromator is led into the detector, which is then recorded by a photodetector, the output of which is amplified and measured on a recorder. The difference in transmitted signal in the presence and absence of the test element is used to calculate absorption.
Calibration curve
A calibration curve is used to calculate the unknown concentration of an element in a solution. Several solutions of known concentrations are used to calibrate the instrument. The absorbance of each known solution is measured, and a calibration curve of concentration vs absorbance is plotted.
The sample solution is introduced into the device, and the absorbance of the element in this solution is measured. The calibration curve is then used to compute the unknown concentration of the element.
Applications of atomic absorption spectroscopy
The Atomic absorption spectrometry technique is one of the most important instruments for detecting trace (ppm and ppb) metallic elements.
This approach is used for the quantitative determination of approximately 60 metals. Atomic absorption spectrometry is useful in all areas of chemical analysis. The atomic absorption spectroscopy method is well-established in analytical chemistry, ceramics, mineralogy, biochemistry, metallurgy, water supply, medicine, and soil analysis.
I. Quantitative analysis
Quantitative analysis depends on determining the amount of radiation absorbed by the sample. This measurement provides the number of absorbing atoms in the light path. However, the concentration of the element in the sample cannot be calculated simply from the number of absorbing atoms in the atomizer. In practice, quantitative measurements are usually dependent on calibration.
Calibration curves are generated using a known concentration solution of the sample element. The sample is atomized similarly to the standard, and its concentration is calculated using the calibration curve. Atomic absorption spectroscopy is used for the quantitative determination of various metal elements.
II. Metallurgical and inorganic analysis ;
The elemental composition of minerals and rocks provides important information on the commercial viability of mining activities in areas that have been studied. After mining, the ores and minerals must be examined for composition for refining activities to be efficient. Similarly, trace metal analysis is extremely useful in the search for oil and water deposits.
Atomic absorption spectrometry has been used to analyze trace metals in geological, biological, metallurgical, glass, cement, engine oil, marine sediments, pharmaceutical, and atmospheric samples. It is also used to determine trace elements in the ground, surface water, rocks, ores, minerals, and so on. Atomic absorption spectroscopy analyzes light alloys almost completely. Atomic absorption spectroscopy is also used to determine Co, Cr, Mg, Mn, Pb, Zn, and other elements in iron and steel.
III. Pollution monitoring
Monitoring for trace metal pollution in the atmosphere and industrial effluents, oceans, rivers, and lakes is critical for determining the safety of living beings.
Atomic absorption spectrometry is utilized in pollution analysis, such as lead in the atmosphere and arsenic in ground water.
IV. Food and Beverages
In the food and beverage industry, it is used for the analysis of various element concentrations in wine, beer, and fruit drinks. Atomic absorption spectroscopy is also used for the determination of various types of contamination in food.
V. Medical pathology
- Ca, Mg, Na, and K levels in blood serum and plasma are determined by using Atomic absorption spectrometry.
•Atomic absorption spectroscopy is used in blood analysis for the determination of Na, K, Fe, Cu, Zn, and Pb.
• Atomic absorption spectroscopy is used in hair and nail analysis for the determination of Co, Zn, Mg, Pb, Cu, and Fe.
- Atomic absorption spectroscopy is utilized in the analysis of metals in food.
- Atomic absorption spectroscopy is utilized for metal analysis in petroleum products and lubricating oils.
VI. Pharmaceutical industry
Trace metal analysis is critical in formulation development, catalyst efficiency, and dose limit determination. Most elements have a helpful role up to specific prescribed limitations, but their impacts are hazardous beyond those limits. In the pharmaceutical industry, atomic absorption spectroscopy is used for the determination of trace elements present in drugs.
VII. Biochemical analysis
Atomic absorption spectroscopy is used to determine Na and K levels in biological fluids, as well as Cd and Zn levels in industrial employees’ urine. In forensic labs, trace metal analysis gives useful information on specimens such as stomach contents for food poisoning, paint chips, fibers, and hair strands gathered at a crime scene.
VIII. For material development
Composition and trace metals have a significant impact on common material characteristics such as hardness, brittleness, grain size, crystallinity, and amorphous nature. Trace metal analysis can provide useful information on the performance attributes of such materials.
IX. Agriculture
Atomic absorption spectroscopy is used to determine the presence of metallic elements in soil such as Ca, Sr, and Co. Additionally, it is utilized in animal feed analysis for Zn, Cu, Mn, Ca, K, Na, and other elements. It is also used for analysis of animal fertilizer to determine the concentration of Ca, Cu, Mg, Fe, and other elements.
X. Selected determination by atomic absorption spectroscopy
- Mg and Ca concentrations in tap water (acetylene air flame)
- Vanadium present in lubricating oil
- Trace elements (Zn, Cu, Ni, Cd) in polluted soil
- Tin in canned fruit juice.
- Chromium in steel.
- Arsenic in soil and water by hydride formation.
- Arsenic in soil and water by hydride generation.
References
- S. K. Gautam, B. R. Poudel, H. R. Sharma (2016). Concise Analytical Chemistry, National Book Centre.
- https://www.sciencedirect.com/topics/neuroscience/atomic-absorption-spectroscopy.
- https://www.slideshare.net/sharmasuriti/atomic-absorption-spectroscopy-15185397.
- https://www.labcompare.com/Spectroscopy/Atomic-Absorption-Spectrophotometer/.
- https://www2.chemistry.msu.edu/courses/cem434/chap9atomabsspec.pdf.
- https://www.iitk.ac.in/che/PG_research_lab/pdf/resources/AAS-GTA-reading-material.pdf.
- https://www.ufjf.br/baccan/files/2011/05/AAS-Varian1.pdf
