Atom is the smallest unit of matter that can be divided without releasing electrically charged particles. It is also the smallest unit of matter with the properties of a chemical element. It is the smallest constituent unit of matter that possesses chemical element properties. As a result, the atom is the fundamental building block of chemistry. Atoms do not exist in isolation; instead, they combine to form ions and molecules, which then combine in large numbers to form the matter we see, feel, and touch.

Interesting Science Videos
History of Atom
Atoms were created 13.7 billion years ago, following the Big Bang. As the hot, dense new universe cooled, conditions became favorable for the formation of quarks and electrons. Quarks combine to form protons and neutrons. It took 380,000 years for the universe to cool enough for the electrons to slow down enough for the nuclei to capture them and form the first atoms. According to Jefferson Lab, the first atoms were primarily hydrogen and helium, which are still the most abundant elements in the universe. Gravity eventually caused clouds of gas to coalesce and form stars, and heavier atoms were (and still are) created within the stars and ejected into space when the star exploded (supernova).
Atomic Model Structure
Scientists’ atomic models have evolved over time as experimental evidence has improved our understanding of atomic structure.
In 1803, John Dalton presented his atomic theory, which was explained on three key concepts:
- Firstly, atoms, which are tiny particles that cannot be created, destroyed, or divided, make up matter.
- Atoms of the same element are the same, while atoms of different elements are not.
- Various atoms combine to form new substances.
The theory was correct at the time, but as science progressed, some aspects of Dalton’s theory were disproved.
Hence, this is a fundamental feature of science: new experimental evidence may lead to the modification or replacement of a scientific model.
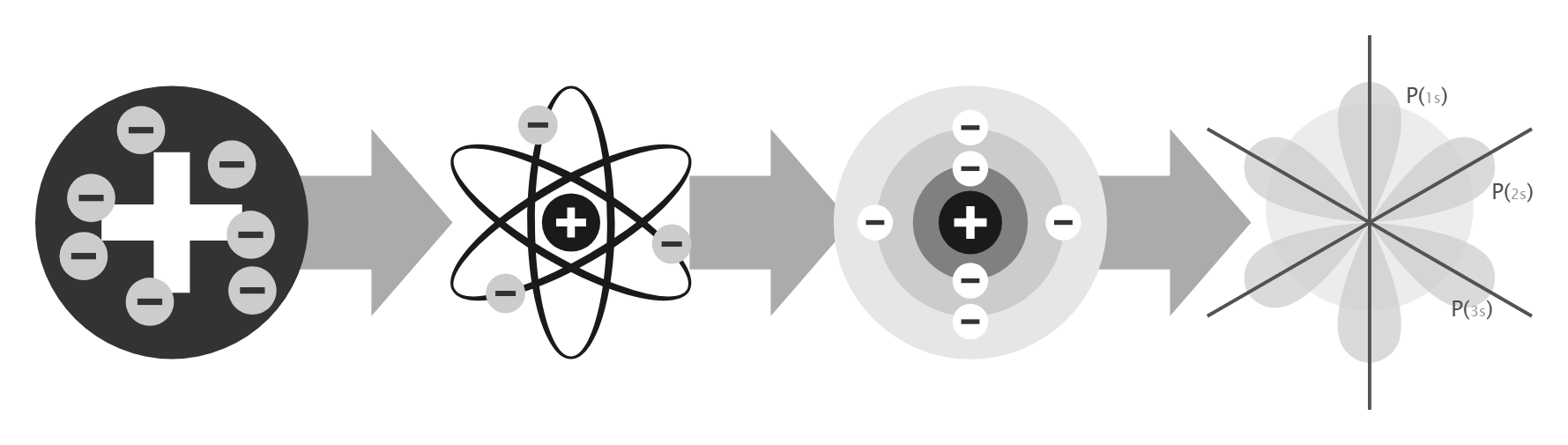
Plum Pudding Model
J.J. Thomson discovered the electron in 1897.
He experimented with a cathode-ray tube that identified the electron as a negatively charged subatomic particle, as well as proving that atoms are divisible.
Thomson proposed the plum pudding model of the atom based on his research, which depicted negative electrons distributed throughout soft globules of positively charged material.
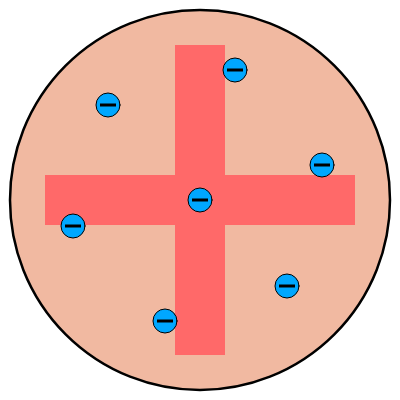
Limitations of the plum pudding model
- It failed to explain atomic stability because his atomic model did not explain how a positive charge holds negatively charged electrons in an atom. As a result, this theory also failed to account for the nucleus’s position in an atom.
- Thomson’s model was unable to account for the scattering of alpha particles by thin metal foils.
- There is no experimental evidence to back it up.
Rutherford’s Atomic Model
Ernest Rutherford presented his atomic model based on the famous gold foil experiment in 1909.
Rutherford fired a beam of positively charged particles at a thin sheet of gold foil, expecting the particles to pass through because the positive charge of the nucleus was thought to be evenly distributed.
However, some particles were scattered and a few were deflected directly back, leading him to hypothesize that the majority of an atom’s mass is concentrated in a region of space at the center of the atom known as the nucleus.
Rutherford’s scattering experiments did not support the idea that atoms behaved as described in the plum pudding model, so the model was abandoned.
According to Rutherford’s model, the atom is mostly empty space, with the nucleus at the center and electrons orbiting in paths around it.
This was referred to as the nuclear model of the atom.
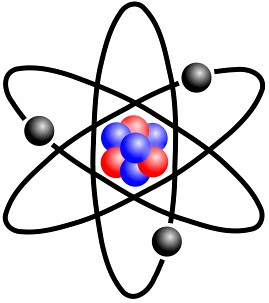
Limitation of Rutherford Atomic Model
Rutherford’s atomic model was experimentally successful and widely accepted by scientists. However, it was unable to explain certain critical aspects of atoms.
- His theory and model were unable to explain the atom’s stability. According to Rutherford’s observations, electrons in an atom move at higher speeds in circular orbits around its nucleus. This behavior contradicted Maxwell’s findings. According to Maxwell, the acceleration of charged particles in atoms causes them to emit electromagnetic radiation. As a result, electrons moving around an atom’s nucleus must constantly emit electromagnetic radiation. These facts were not explained by Rutherford’s atomic model.
- Because of their kinetic motion, electron electromagnetic radiations have energy. It will, however, cause their orbit to contract as they continue to emit energy and collapse in the nucleus. And, according to Maxwell’s theory, this will happen in 10-8 seconds. As a result, we can conclude that Rutherford’s model contradicts Maxwell’s theory and fails to explain atomic stability.
- Rutherford’s atomic model is incomplete because it does not explain any arrangement of electrons in orbit. It is one of the most serious flaws in Rutherford’s atomic model.
- Positively charged particles make up an atom. The majority of this positive charge is concentrated in a small region of the atom known as the nucleus. This Rutherford model hypothesis was later debunked by the discovery of protons and neutrons.
- Many negatively charged particles known as electrons surround the atom’s nucleus. In circular orbits, these electrons move faster around the nucleus. Later research, once again, disproved this assumption.
Bohr Atomic Model
Niels Bohr expanded on the nuclear model in 1913, proposing that electrons orbit the nucleus in fixed shells or orbitals located at predetermined distances from the nucleus.
Each orbital has a different energy, with the higher energy orbitals being further away from the nucleus.
This model explained why the atom does not collapse inwards due to the attraction between the positive nucleus and the negative electrons that circle it.
Bohr’s theory and calculations matched the experimental findings.
Further research and testing revealed that the nucleus could be divided into smaller particles, each with the same mass and charge.
This research resulted in the discovery of the proton.
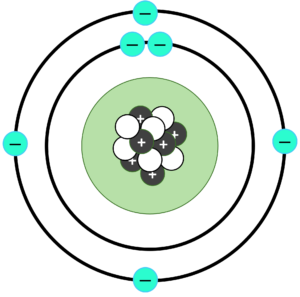
Limitations of Bohr atomic model
- One of the limitations of the Bohr model is that it cannot explain the splitting of spectral lines when they are influenced by an electric field (Stark effect) and a magnetic field (Zeeman Effect). It did not explain how atoms can form molecules through chemical bonds.
- The electrons revolving around the nucleus are considered on a planar dimension in the Bohr plan. This is one of the most significant limitations of the Bohrs model. The dimension in which the electron moves around the nucleus was later discovered to be three dimensional.
- The most significant limitation of Bohr’s model was its inability to predict large-sized atoms and explain the spectrum of atoms with only two electrons, such as the helium atom.
- Another limitation of the Bohr model was the emphasis on hydrogen. It also violated Heisenberg’s Principle. This principle states that it is impossible to determine the precise position and momentum of electrons at the same time. Electrons in Bohr’s model revolve in a well-defined circular orbit.
Quantum Mechanical Model
One major flaw in Bohr’s model was that it treated electrons as particles with precisely defined orbits. Erwin Schrödinger, an Austrian physicist, proposed that the behavior of electrons within atoms could be explained mathematically by treating them as matter waves, based on de Broglie’s idea that particles could exhibit wavelike behavior. This model, which is the foundation of modern atomic understanding, is known as the quantum mechanical or wave mechanical model.
Size of an Atom
An atom’s size is extremely small, much smaller than our imagination. When millions of atoms are stacked together, a layer of an atom the thickness of a thin sheet of paper is formed.
Whether they have three or ninety electrons, all atoms are roughly the same size. A row of approximately 50 million atoms of solid matter would measure 1 cm (0.4 inches). The angstrom(Å), defined as 10-10 meters, is a useful length unit for measuring atomic sizes. A single atom has a radius of (1-2) Å. The nucleus is even smaller in comparison to the overall size of the atom. It is proportional to the atom in the same way that marble is to a football field. The nucleus occupies only 10-14 meters of space in the atom or one part in 100,000. Because it is difficult to locate the positions of electrons surrounding the nucleus, measuring the size of an isolated atom is impossible. However, the radius of an atom can be estimated by assuming that the distance between adjacent atoms is half its radius.
Atomic Mass
It refers to the mass of an atom in a chemical element. Atomic mass roughly corresponds to the total number of neutrons and protons in the atom. It is measured in atomic masses (denoted by u). 1 amu is 1/12th of the mass of one C-12 atom, and the relative atomic masses of elements are determined concerning the C-12 atom.
Subatomic Particles
An atom is made up of three particles: neutrons, protons, and electrons, with the exception of hydrogen, which has no neutrons.
- Every atom has a nucleus that is surrounded by one or more electrons.
- The nucleus typically contains a similar number of protons and neutrons, which are referred to collectively as nucleons.
- Protons have a positive charge, electrons have a negative charge, and neutrons have no charge.
Protons
Ernest Rutherford discovered protons in 1919 while conducting his gold foil experiment. He deflected positive alpha particles by projecting alpha particles (helium nuclei) at gold foil. He came to the conclusion that protons exist in nuclei and have a positive nuclear charge. The atomic number, also known as the proton number, is the number of protons in an atom. An element’s atomic number is determined by its atomic number (e.g., the element of atomic number 6 is carbon).
The mass of a proton is 1.676 * 10-24 grams.
The charge of a proton is +1.602 * 10-19 Coulombs.
Electrons
Sir John Joseph Thomson discovered electrons in 1897. J.J. Thomson demonstrated the mass-to-electric-charge ratio of cathode rays after many experiments with them. He established that cathode rays are fundamental negatively charged particles; these cathode rays became known as electrons. Robert Millikan discovered the value of the electronic charge through oil drop experiments.
Electrons are found in an electron cloud, which is the area surrounding the atom’s nucleus. The likelihood of finding an electron near an atom’s nucleus is usually higher. The symbol for electrons is e–. Electrons have a negative charge that is proportional to the positive charge of protons. Their mass, however, is significantly less than that of a proton or neutron (and as such is usually considered insignificant). Ions are formed when protons and electrons are in unequal amounts, resulting in positive cations or negative anions.
When compared to the mass of a proton, the mass of an electron is negligible. It has a mass that is equal to (1/1837) times the mass of a proton.
An electron’s charge is equal to -1.602 * 10-19 Coulombs.
Neutrons
James Chadwick discovered neutrons in 1932 when he demonstrated that penetrating radiation contained beams of neutral particles. Neutrons and protons coexist in the nucleus. They, along with protons, account for nearly all of the atom’s mass. The neutron number is the number of neutrons found by subtracting the proton number from the atomic mass number. The neutrons in an element determine an atom’s isotope and, in many cases, its stability. The number of neutrons does not always equal the number of protons.
The mass of a neutron is 1.676 * 10-24 grams.
Some other atomic particles are:
Alpha Particles
Alpha particles are denoted by He2+. Helium nuclei are made up of two protons and two neutrons. An alpha particle has no net spin. They are produced by the alpha decay of large, unstable atoms. The process by which an atom emits an alpha particle and thus becomes a new element is term as alpha decay. This only happens with elements that have large, radioactive nuclei. Tellurium, element 52, is the smallest element that emits alpha particles till today. In general, alpha particles are not harmful. A single sheet of paper or one’s skin can easily stop them. They can, however, cause significant damage to one’s internal organs. Alpha decay is applicable as a safe source of energy for radioisotope generators.
Beta Particles
Beta particles (β) are high-energy free electrons or positrons that are emitted in a process known as beta decay. Positrons are positively charged and have the same mass as electrons. There are two types of beta decay: electron emission and positron emission. Household items such as wood or an aluminum plate or sheet can stop beta particles, which are 100 times more penetrating than alpha particles. Beta particles can penetrate living matter and can sometimes change the structure of molecules they collide with. The change is usually considered damage, and it can lead to cancer and death. In contrast to their harmful effects, beta particles can be used in radiation therapy to treat cancer.
References
- https://www.britannica.com/science/atom
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/1-physical-chemistry/1-1-atomic-structure/1-1-1-particles-in-the-atom–atomic-structure/
- https://byjus.com/chemistry/subatomic-particles/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Atomic_Theory/The_Atom/Sub-Atomic_Particles
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura.General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall, 2007.
- https://www.britannica.com/science/atom/Atomic-bonds
