Interesting Science Videos
What is Asparagine?
Asparagine is a non-essential, alpha-amino acid that was first isolated in 1806 from asparagus juice by Vauquelin and Robiquet.
They discovered asparagine after they observed cubic crystals in the sap of asparagus in 1802 by Delaville. The first amino acid discovered is asparagine. It is the constituent of proteins that is found in the free state of plants and animals. It plays an important role in the biosynthesis of glycoproteins and other proteins that is a beta-amido derivative of aspartic acid. It is a non-toxic carrier of ammonia that carries ammonia from the body. Asparagine is a non-essential amino acid as the body can produce itself; alanine, cysteine is one of them. Asparagine is also a glucogenic amino acid that is produced by the liver.
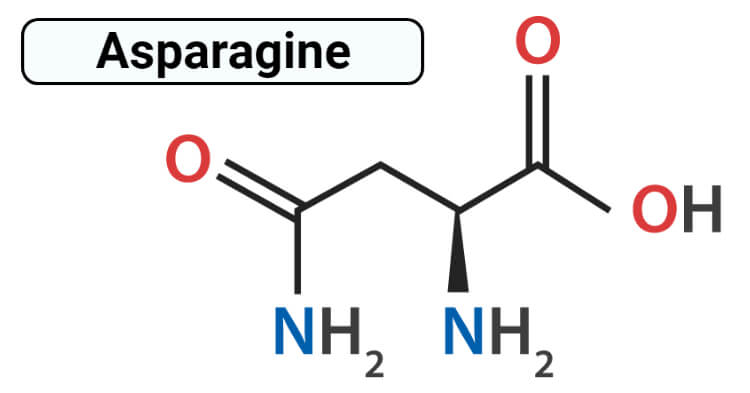
Structure of Asparagine
Asparagine has an optically active form having L-configuration that is L-asparagine. It consists of an alpha carboxyl group, alpha-amino group and has a side chain consisting of polar aliphatic amino acids. Asparagine is the amide of aspartic acid that doesn’t carry a formal charge at any pH conditions. The amide group of asparagine can donate while two hydrogen bonds while the carbonyl group acts as a hydrogen bond acceptor and is found on the surface and also deep inside within proteins. Asparagine lacks a negative charge of the carboxylic acid group of aspartate and withholds polarity and also plays a major role in the site of enzymes.
Sources of Asparagine
They are found in huge amounts in plant sources like legumes, soy, nuts, wheat, potatoes. Asparagine that is found in animal sources is seafood, fish, eggs, dairy products, lactalbumin, etc. They are also found in roasted coffee and French fries.
Physical properties of Asparagine
- White in color with a crystalline appearance
- Polar
- Uncharged
- Dry powder, solid
- Orthorhombic bisphenoidal crystals
- Flammable in natur
Chemical properties of Asparagine
- The molecular formula is C4H8N2O3.
- Molecular weight: 132. 12
- Neutral
- Melting point: 234-235ºC
- Boiling point: 438ºC
- Insoluble in methanol, ethanol, ether, and benzene
- Soluble in both acid and alkali but moderately soluble in water
- N:C ratio of asparagine is 2:4
- pKa: 8.82
- Solubility: 29400 mg/L at 25ºC
- Isoelectric point: 5.41
Asparagine Biosynthesis
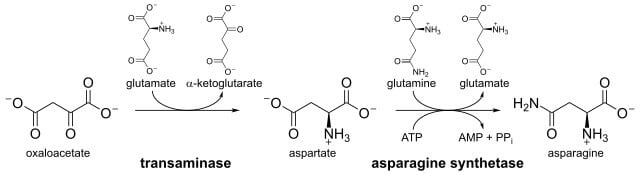
In previous studies, it was found that aspartate synthesis occurs by amidation of aspartate by a reaction that is similar to that catalyzed by glutamine synthetase. But it was later found that asparagine is synthesized from aspartic acid and ammonia by the enzyme asparagine synthetase. The whole reaction that occurs is ATP-dependent amidotransferase reactions. Oxaloacetate in transamination is the primary component in the biosynthesis of asparagine from which the whole process starts. Oxaloacetate is catalyzed by aspartate aminotransferase 1. L-asparagine is converted from L-aspartate in a reaction catalyzed by the enzyme asparagine synthetase that uses L-glutamine as an amide donor. Magnesium ions and Adenosine Triphosphate (ATP) are required for this reaction that involves the formation of a beta-aspartyladenylate intermediate which is then converted to L-asparagine. In this process, ammonia is transferred from L-glutamine to produce l-glutamate and AMP. Asparagine synthetase in humans is responsible for cellular stress because of transcription caused by a gene located on chromosome 7.
Functions and Uses of Asparagine
- As asparagine is synthesized from aspartate and glutamine, it is a dispensable amino acid that has major three functions:
- It is the major source of amino acid that is used in the production of other dispensable amino acids through the enzyme transaminase.
- It is the precursor that is required for the synthesis of DNA, RNA, and ATP.
- It can increase cellular energy production by contributing carbon chains to the citric acid cycle.
- It acts as a diuretic.
- It helps in controlling metabolic activities of the brain and helps in maintaining equilibrium.
- Used as supplemental nutrition for treatment of dietary deficiency and for physically imabalanced peoples.
- Mutation in asparagine synthetase leads to disability intellectually and physically.
Safety and Toxicity of Asparagine
- Excess consumption of asparagine can cause leukemia and lymphomas as asparaginase acts as an anti-cancerous agent and is used in treating tumors.
- Deficiency of asparagine leads to irritability, headaches, depression, confusion and psychosis.
References
- From, https://assets.speakcdn.com/Assets/2606/0e2040343_asparagine-supplemental-information-page.pdf.
- From, https://www.britannica.com/science/asparagine
- From, https://pubchem.ncbi.nlm.nih.gov/compound/Asparagine
- From, https://byjus.com/chemistry/asparagine-amino-acid/
- From, https://biocyc.org/HUMAN/NEW-IMAGE?type=PATHWAY&object=ASPARAGINE-BIOSYNTHESIS
- From, http://www.biology.arizona.edu/biochemistry/problem_sets/aa/asparagine.html
- Lea PJ, Sodek L, Parry MAJ, Shewry PR and Halford NG (2007). Asparagine in plants. Ann Appl Biol. 1-26
- Meister A (1974). Asparagine Synthesis. The Enzymes. 561–580. doi:10.1016/s1874-6047(08)60149-3 .
