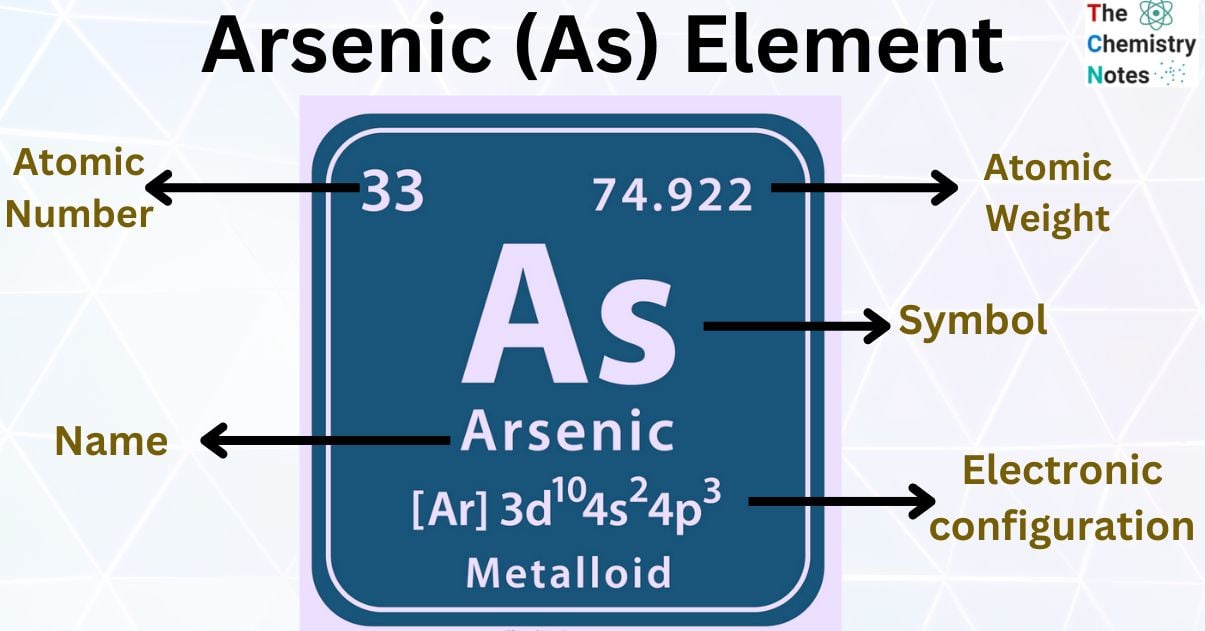
Arsenic is the metallic element with the atomic number 33 and is represented by the symbol ‘As’ in the periodic table. It is classified as a metalloid and belongs to the p-block of group 15 of the periodic table. It is a brittle silvery-grey semi metal. It has three allotropic forms yellow, black, and grey.
Arsenic is the 53rd most abundant element on Earth’s crust. It makes up around 0.00015 percent of the crust. But its occurrence in free nature is rare. Typically, the element can be found in minerals such Arsenopyrite (FeAsS), realgar (As4S4), and orpiment(As2S3).
History of Arsenic
- Arsenical bronze which was a hard alloy made of bronze included arsenic during the bronze age.
- Humans have long employed arsenic for many purposes. It was notorious for being used to assassinate members of the royal family as a means to gain power and thrones. It earned a reputation as the “king of poison” as a result.
- Because of its role in executing family members, arsenic was referred to as “inheritance powder” during the Renaissance Era.
- Jabir ibn Hayyan, an alchemist and author from Iraq was the first to describe the isolation of arsenic in his book prior to the year 815 AD.
- In 1250, Albertus Magnus, a German scientist also known as Albert the Great, isolated the element from a compound by heating soap together with arsenic trisulfide.
- James Marsh, an English chemist, was able to develop a sensitive and specific chemical test for the arsenal (Marsh test) in 1836, which made the detection of arsenic poisons used for murders simpler.
- Arsenic was used as an insecticides in the agriculture during the mid 1800’s.
- The word “arsenic” comes from the Latin “arsenicium” and the Greek “arsenikos,” both of which mean “masculine” or “male” in the days when people believed that metals had various sexes.
Occurrence of Arsenic
- Natural Arsenic comprises roughly 0.00015 % of the Earth’s crust. It does not occur frequently in a free state and is found in combination with iron, sulfur, and oxygen. It is generally found in sedimentary rocks at much higher concentrations than in any other type. It is also associated with sulfide deposits.
- Arsenic is mostly gathered from copper processing dust and can also be found in smelting dust from copper, gold, and lead smelters.
- China generated more than 70% of the white arsenic used in 2014, followed by Morocco, Russia, and Belgium.
Isotopes of Arsenic
Arsenic is a monoisotopic element with only one stable isotope which is 75As.
Naturally occurring isotopes
| Isotope | Natural abundance (atom %) |
|---|---|
| 75As | 100 |
Elemental Properties of Arsenic
| Electronic Configuration | [Ar] 3d104s24p3 |
| Atomic Number | 33 |
| Atomic Weight | 74.922 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 15, 4, p-block |
| Density | 5.75 g.cm -3 at 20 °C |
| Ionic radius | 0.222 nm (-2) 0,047 nm (+5) 0,058 (+3) |
| Van der Waals radius | 0.139 nm |
| Electron shells | 2, 8, 18, 5 |
| Electrons | 33 |
| Protons | 33 |
| Neutrons in most abundant isotope | 42 |
Physical Properties of Arsenic
Arsenic has an atomic number of 33 and is a hard silvery metal. It has a melting point of 814°C , and a boiling point of 1135 °F (point of sublimation).
The melting point quoted above is for gray arsenic under a pressure of 28 atmospheres.
NOTE: At normal atmospheric pressure arsenic does not melt when heated, it sublimes. i.e. when heated, arsenic undergoes a phase change directly from solid to gas.
- The density of Arsenic is 5.7 grams per cubic centimeter at 14°C.
- Arsenic has three allotropes : yellow, grey, and black, grey being the most common.
- Arsenic and its compound are toxic.
- It is brittle solid and is not a good conductor of heat and electricity.
- Arsenic is odorless and tasteless.
| Color/physical appearance | gray |
| Melting point/freezing point | 1135 °F (Sublimes) |
| Boiling point | 1135 °F (sublimes) |
| Density | 5.75 g cm-3 at 20°C |
| Malleability | No |
| Ductility | No |
Chemical Properties of Arsenic
Arsenic can tarnish quickly in air, in its elemental state and it can emit a form of grayish or white arsenic trioxide cloud at higher temperatures. Non-metallic form of arsenic does not seem to react as much but it will dissolve in alkalis and concentrated acids if heated.
Chemical Reaction of Arsenic
- Reaction of Arsenic with Water
In the absence of oxygen, Arsenic does not react with water.
- Reaction of Arsenic with Air
Arsenic’s surface oxidizes in moist air, turning the metal into a bronze tarnish that later turns black. In dry air, arsenic does not react. Arsenic ignites “arsenic trioxide” and, in certain cases, “tetraarsenic hexoxide,” As4O6, when it burns in air. When heated, arsenic releases “arsenic pentoxide” (tetraarsenic decaoxide, As4O10, and As4O6), which burns in oxygen.
4As(s) + 5O2(g) → As4O10(s)
4As(s) + 3O2(g) → As4O6(s)
- Reaction of Arsenic with the Halogens
When Fluorine (F2), and Arsenic reacts ; it forms a gas called pentafluoride arsenic(V) fluoride
2As(s) + 5F2(g) → 2 AsF5(g) [colorless]
Arsenic reacts under controlled conditions with the halogen fluorine (F2) to form the trihalide arsenic (III) fluoride, AsF3.
2As(s) + 3F2(g) → 2AsF3(l) [colourless]
Arsenic reacts under controlled conditions with the halogen chlorine (Cl2) to form arsenic (III) chloride, AsCl3.
2As(s) + 3Cl2(g) → 2AsCl3(l) [colourless]
Arsenic reacts under controlled conditions with the halogen bromine (Br2) to form arsenic (III) bromide, AsBr3.
2As(s) + 3Br2(g) → 2AsBr3(s) [pale yellow]
Arsenic reacts under controlled conditions with the halogen iodine (I2) to form arsenic (III) iodide, AsI3.
2As(s) + 3I2(g) → 2AsI3(s) [red]
Uses Of Arsenic
Arsenic and its compounds have been produced and used commercially for centuries. They are used in variety of other industrial purposes which include:
Used In Medicinal Purpose: Throughout the 20th century, arsenic was used as a common ingredient in many medicines, but as its lethal qualities and adverse effects came to light, its use in medicines gradually declined. Melarsoprol is still used to treat trypanosomiasis. Arsenic trioxide may be used to treat people with acute promyelocytic leukemia. Recent research has used 74As to locate malignancies. Because cancer cells can be killed by nanoparticles.
Used As An Alloy: Arsenic element is used to create alloys, especially those containing copper and lead. Arsenic, along with various metals including non-metals, is able to create several alloys. Gallium arsenide and arsine are commonly utilized in the semiconductor and electronics industries.Gallium arsenide is a material that is utilized in high-speed semiconductor devices, high-power microwave, and millimeter-wave devices, and optoelectronic devices, such as fiber-optic sources and detectors, due to its high electron mobility as well as its light-emitting, electromagnetic, and photovoltaic capabilities.
Used For Agricultural Purposes: Arsenic is mostly utilized to keep wood from degrading. Arsenic is used to treat wood considering it is poisonous and has a strong antimicrobial effect on germs, fungi, and insects. Arsenic is used to eliminate pests that destroy crops, such as rats, fungi, insects, and others. Numerous poisons and insecticides used on farms contain it as an ingredient. The production of pigs and birds is also done using it. Chicken feed contains organic arsenic compounds because they are safer than elemental arsenic and help chicks grow.
The leather tanning business also uses arsenic as a preservative. It is frequently sprayed over fields from a light aircraft to eradicate any form of infestation and is widely used as a weed killer. Due to its success in eliminating termite diseases, it is also employed as an insecticide. However, the amount of arsenic used for agricultural purposes has significantly decreased as organic pesticides gain popularity.
Health Effects Of Arsenic
- Inorganic arsenic is extremely poisonous. The greatest concern to human health from arsenic is contaminated water used for drinking, food preparation, and crop irrigation.
Long-term arsenic exposure through drinking water and food can result in cancer and skin problems. - It’s also linked to cardiovascular disease and diabetes. In utero and early childhood exposure has been linked to poor cognitive development and an increase in young adult mortality.
Heavy chronic exposure causes patchy skin darkening, tiny focal keratoses, and other skin lesions.
Arsenic’s gastrointestinal effects (GI) are typically the result of ingestion; but, GI symptoms can arise after significant exposure via other routes. - Arsenic is a metalloid that may be present in the groundwater. Only if a large amount is ingested it could be fatal.
- Arsenic has the potential to harm the kidneys.
- Acute tubular necrosis with acute renal failure may occur as a result of systemic toxicity in severe acute arsenic poisoning.
Environmental Effects of Arsenic
Arsenic’s toxicity in mammals varies depending on various aspects, including its form (organic or inorganic), valence state, rate of absorption and subsequent elimination, solubility, and particle size.
- The aquatic and terrestrial biota exhibit a wide spectrum of arsenic sensitivity. Abiotic and biological variables influence their sensitivity.
- The fundamental mechanism of arsenite toxicity is thought to be binding to protein sulfhydryl groups. Arsenate has been shown to inhibit oxidative phosphorylation by competing with phosphate.
- Arsenate toxicity to biota is often minimized in situations with high phosphate concentrations. Because arsenate is a phosphate analogue, organisms living in high arsenate environments must obtain phosphorous while avoiding arsenic toxicity.
- Arsenic is mostly ingested through food and drinking water, with food being the most important source in most cultures.
- Animals are exposed to arsenic via contaminated drinking water, feed, grasses, vegetables, and other leaves.
- Arsenic is one of the most prevalent inorganic chemical poisonings in agricultural animals. Although arsenic exposure does not usually cause external signs or symptoms in farm animals, arsenic (or metabolite) concentrations in blood, hair, hoofs, and urine remain high in animals from arsenic-contaminated areas. As a result, it is presumed that arsenic concentrations in blood, urine, hair, or milk have been employed as biomarkers of arsenic exposure in field animals.
Arsenic Poisoning
Arsenic poisoning is a worldwide public health problem that affects millions of individuals through environmental and occupational exposure, as well as purposeful suicide and homicide attempts.
Arsenic (As) is a hazardous metalloid element that is practically tasteless and odorless and is found in abundance in the environment. Arsenic is found in four common valence states: As(o), As(III), As(V), and Arsine gas, as well as three different forms: inorganic salt, organic salt, and gaseous form.
Arsenic poisoning can cause the following immediate symptoms:
- Pain in the abdomen.
- Vomiting and nausea.
- Diarrhea.
- Cough.
- Chest ache.
- Dyspnea (shortness of breath).
- Pharyngitis (inflammation of the throat).
- Arrhythmia (abnormal cardiac rhythm).
- Hypotension (low blood pressure).
- The sensation of “pins and needles” in your fingertips and toes.
- Skin that is red and inflamed.
- Garlic smell in your breath and tissues.
Watch out for the interesting videos about the Arsenic Element.
References
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. ISBN 0-8493-0464-4.
- https://www.rsc.org/periodic-table/element/33/arsenic
- https://www.lenntech.com/periodic/elements/as.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/Arsenic#section=Melting-Point
- An insight of environmental contamination of arsenic on animal health. https://doi.org/10.1016/j.emcon.2017.01.004
- Emsley, John (2011). “Arsenic”. Nature’s Building Blocks: An A–Z Guide to the Elements. Oxford, England: Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Rieuwerts, John (2015). The Elements of Environmental Pollution. Abingdon and New York: Routledge. ISBN 978-0-41-585920-2.
- Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. https://doi.org/10.1016/j.hazadv.2022.100052
- https://my.clevelandclinic.org/health/diseases/24727-arsenic-poisoning
- Arsenic Toxicity: Matthew Kuivenhoven1; Kelly Mason2.

