The Arrhenius equation holds significant importance within the field of physical chemistry. The Arrhenius equation is a mathematical expression that elucidates the relationship between temperature and the rate of chemical reactions.
The Arrhenius equation is widely utilized for determining the rates of chemical reactions across a broad range of applications. Additionally, it plays a crucial role in the computation of the activation energy.
The equation was formulated by Svante Arrhenius in 1889. The formulation of the van ‘t Hoff equation, which accounts for the temperature dependence of equilibrium constants, is attributed to the Dutch chemist Jacobus Henricus Van’t Hoff.
What is the Arrhenius Equation?
The Arrhenius equation is a mathematical formula that correlates the activation energy and temperature of a reaction with the rate constant, k. It’s significant since it reveals the relationship between temperature and reaction rate.

Arrhenius Equation: Expression
The Arrhenius equation expression is shown as:
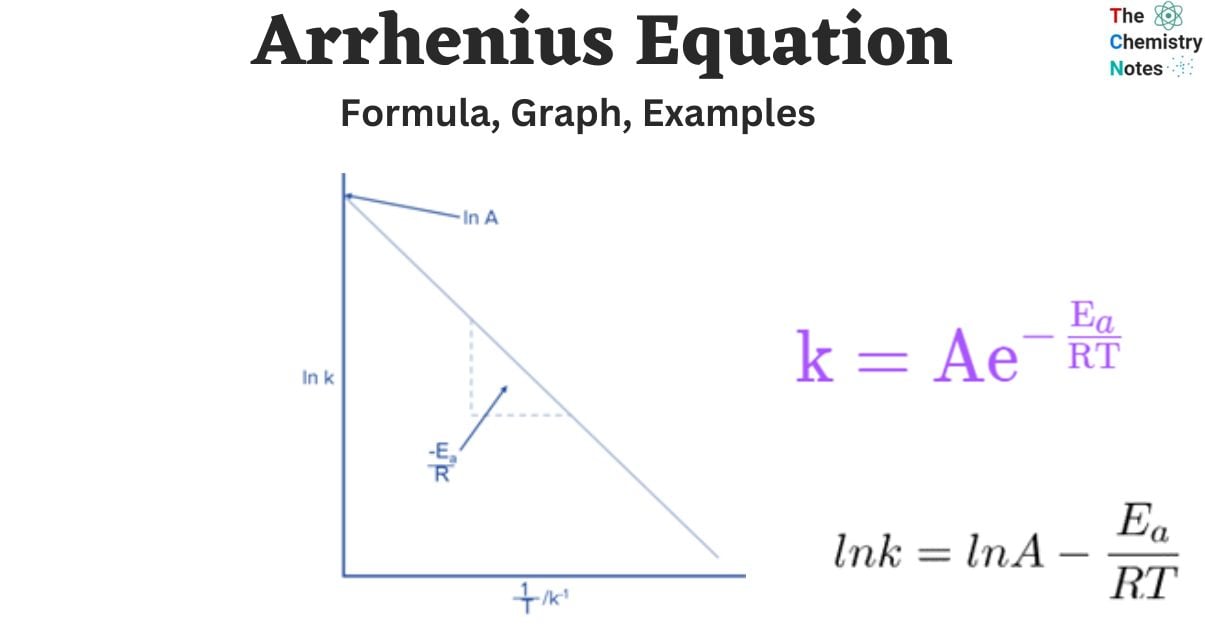
k=Ae-Ea/RT
Where,
- k denotes the rate constant of the reaction
- A denotes the pre-exponential factor. In terms of the collision theory, it is the frequency of correctly oriented collisions between the reacting species
- e is the base of the natural logarithm (Euler’s number)
- Ea signifies the activation energy of the chemical reaction (energy per mole)
- R denotes the universal gas constant
- T denotes the absolute temperature associated with the reaction (in Kelvin)
The above equation may also be written as follows when the energy is taken as energy per molecule of the reactants.
k = Ae-Ea / kbT
where
kb represents the Boltzmann constant.
Derivation of the Arrhenius Equation
From experimental data, the Arrhenius equation can be deduced. When the equation is linearized into the form y=mx+c, we obtain
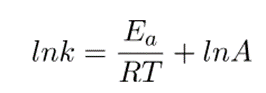
This equation can be arranged as;
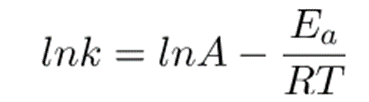
Removing natural log;
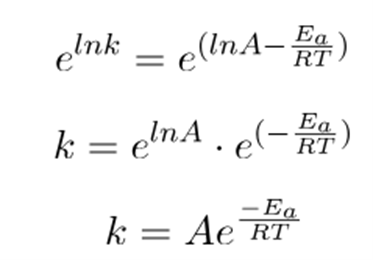
In temperature conditions, the equation becomes.
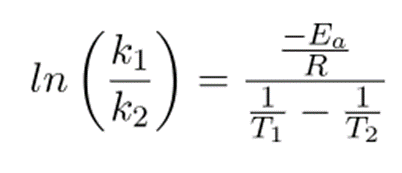
The Arrhenius Equation and Pre-Exponential Factor (A)
The letter ‘A’ in the Arrhenius equation represents the preexponential factor or frequency factor. This factor relates to collisions between molecules and can be viewed as the frequency of correctly oriented collisions between molecules with sufficient energy to initiate a chemical reaction.
Frequently, the following equation represents the pre-exponential factor:
A = ⍴Z
Where Z is the frequency factor (collision frequency) and is the steric factor (deals with molecular orientation).
The value of A must be determined experimentally due to the fact that it assumes distinct values for various reactions. It also depends on the temperature at which the reaction is occurring.
The units of A depend on the sequence of the reaction. For instance, the value of ‘A’ for a second-order rate constant is given in L.mol-1.s-1 (or M-1s-1, since M = mol.L-1), and that for a first-order rate constant is given in s-1.
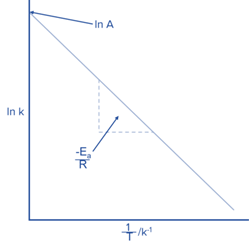
Graph of Arrhenius Equation
Increasing the temperature of a reaction increases the value of the rate constant. The Arrhenius Equation shows the effect of temperature on the value of k.
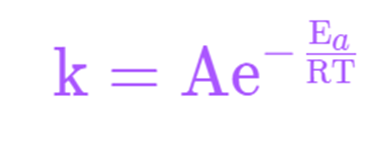
The rate constant likewise grows exponentially with temperature, as expected. This expression, when plotted, will produce a steeply sloping line as the temperature increases.
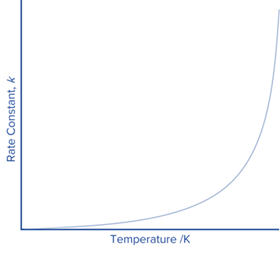
This graph illustrates the Arrhenius equation’s link between the rate constant and temperature, although it would be difficult to use it to find any of the constants.
By plotting the previous equation,
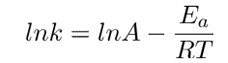
we can determine the slope which will be -Ea/R, and the Arrhenius constant A, which will be the y-intercept.
Arrhenius Equation Applications
- You can use the Arrhenius Equation to determine the ideal temperature for the highest yield.
- The activation energy can be determined from the reaction rate using this method.
Video on Arrhenius Equation
References
- https://protonstalk.com/chemical-kinetics/arrhenius-equation/
- https://mmerevise.co.uk/a-level-chemistry-revision/the-arrhenius-equation/
- https://shiken.ai/chemistry/arrhenius-equation
- https://www.geeksforgeeks.org/arrhenius-equation/
- https://unacademy.com/content/neet-ug/study-material/chemistry/arrhenious-equation/
- https://chemistrytalk.org/arrheniusequation/#:~:text=The%20Arrhenius%20equation%20is%20used,Arrhenius%20equation%20is%20your%20friend.
- https://collegedunia.com/exams/arrhenius-equation-expression-graph-explanation-chemistry-articleid-647#1
- https://byjus.com/chemistry/arrhenius-equation/
- https://www.ibchem.com/IB16/06.41.htm

