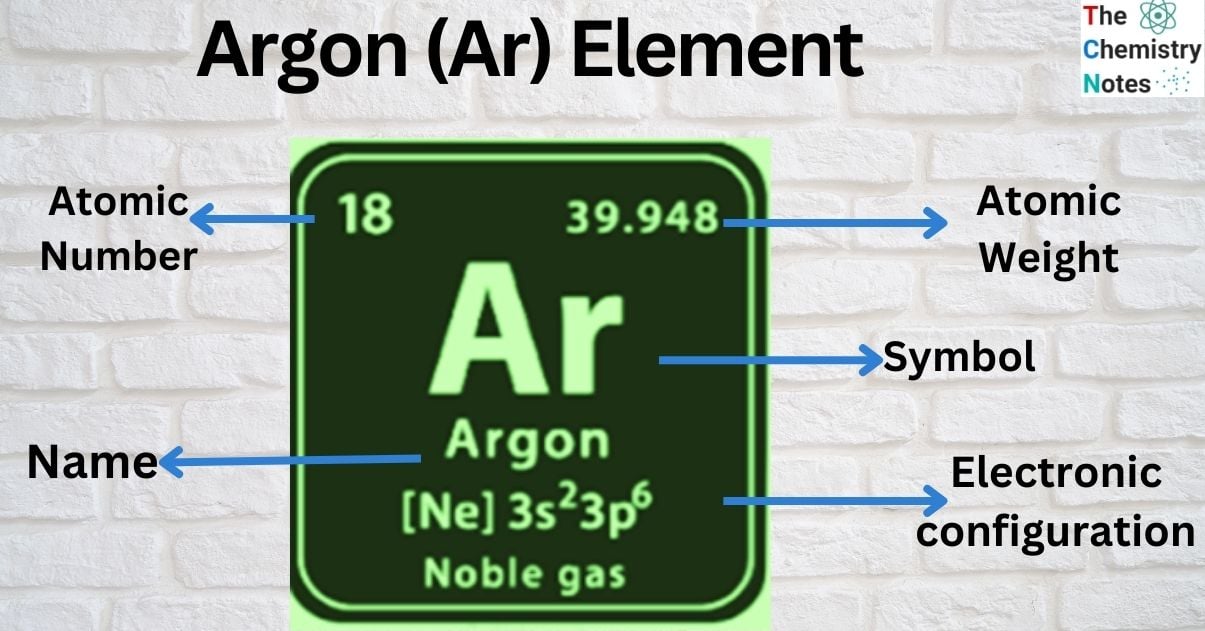
Argon is a chemical element represented by symbol (Ar). Atomic number of argon is 18. It is in the group 18 of the periodic table and it’s a noble gas which comes after Chlorine in periodic table. The name of element Argon is derived from the word “argos” meaning idle as a reference to the element’s nearly complete lack of chemical reactions. Argon is the third most abundant gas on the Earth’s atmosphere. Industrially, argon is extracted by means of fractional distillation of liquid air.
Interesting Science Videos
History of Argon
Argon was the first noble gas discovered.
Sir Henry Cavendish, an English physicist, gave the first clue of its existence in 1785. Cavendish was dissatisfied with how little was known about air. He was particularly dissatisfied with the absence of information on the fraction of air (the majority) that was Oxygen.
It was the year 1894, when argon was first isolated from the air as the gas by
Lord Rayleigh and Sir William Ramsay at the University College London, by removing Oxygen, Carbon dioxide, Water and Nitrogen from a sample of clean air.
This was firstly accomplished by replicating the experiment of Henry Cavendish.
Prior to 1957, the letter ‘A’ was used as the chemical symbol for Argon. IUPAC, on the other hand, agreed to replace the sign in 1957. The gas has a new symbol, ‘Ar.’
Occurrence of Argon
- Argon is the most abundant noble gas on the planet. It is the third most common element in the atmosphere. It accounts for around 1% of the total. Cosmic ray activity creates radioactive Ar-39 in the Earth’s atmosphere. The Earth’s crust contains traces of argon.
- It is more than twice as abundant as water vapor.
- Almost majority of the argon in the atmosphere is radiogenic argon-40, which is formed by the decay of potassium-40 in the Earth’s crust.
- Argon is obtained commercially by the distillation of liquid air.
Isotopes of Argon
- There are 24 known isotopes of argon, with mass numbers ranging from 30Ar to 53Ar. Three of these isotopes, 36Ar, 38Ar, and 40Ar, are stable and naturally exist.
- Argon also has six radioactive isotopes. Argon isotopes are employed in the manufacture of radioisotopes as precursors.
- Ar-40 and Ar-38 are combined to create radioactive K-38, which can be utilized as a blood flow tracer. Ar-40 is used to create radioactive Ar-41, which is used to track gas movements. The radioactive isotopes of Ar-39 and Ar-40 are used in radioactive dating. These are used in determining the ages of rocks, ice ages, igneous rocks, groundwater, etc.
Elemental Properties of Argon
| Electronic Configuration | [Ne] 3s23p6 |
| Atomic Number | 18 |
| Atomic Weight | 39.948 g.mol -1 |
| Standard State | Gas at 298 K |
| Group, Period, and Block | 18, 3, p-block |
| Density | 00.001784 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | 0.192 nm |
| Electron shells | 2, 8, 8 |
| Electrons | 18 |
| Protons | 18 |
| Neutrons in most abundant isotope | 22 |
Physical Properties Of Argon
- Argon is a colorless, odorless, and tasteless gas.
- The density of argon is 1.784 grams per liter. In instance, the density of air is around 1.29 grams per liter which means argon is heavier than air.
- At -185.86°C (-302.55°F), argon transforms from a gas to a liquid. At -189.3°C (-308.7°F) argon transforms from a liquid to a solid.
- Argon has low thermal conductivity.
- Under extreme conditions, argon can form certain compounds even though it is a gas.
- It is characterized by same solubility level in water as that of oxygen.
| Physical State | Gas |
| Elemental Classification | Non-metal |
| Melting Point | −189.34°C, −308.81°F, 83.81 K |
| Boiling Point | −185.848°C, −302.526°F, 87.302 K |
| Density | 0.001633 g cm-1 |
| Atomic Weight | 39.948 |
| Ionization Energies | 1st: 1520.6 kJ/mol 2nd: 2665.8 kJ/mol 3rd: 3931 kJ/mol |
Chemical Properties of Argon
Argon is chemically inactive. It doesn’t even respond at high temperatures or under any other unusual circumstances. On rare occasions, and under extreme conditions, it forms weak, compound-like structures. Under extremely low temperatures, just one argon compound was successfully synthesized.
Uses of Argon
Argon is often used when items must be protected from oxygen or other gases. Argon is a popular element used in the welding industry because it creates an inert environment in which welded metals do not oxidize.However, argon gas can be used in a wide range of industries for a number of applications.
Uses in Lighting
Argon is utilized in neon lighting tubes. When electricity is transmitted through argon, a purple-blue light is produced. It begins to produce light at a considerably lower voltage as it charges. Because it saves money, it soon became the favored gas for this purpose.This is also true with fluorescent illumination. Argon is used in incandescent light bulbs to prevent the filament from oxidizing too quickly. This extends the bulb’s life.
Manufacturing Industry
- Argon lowers chromium losses during steel production in a converter, allowing the target carbon content to be attained at a lower and lower temperature.
- Argon is also employed as a hydrogen removal and degasification agent in the aluminum production process.
- Argon is also used in welding. The technique of joining two metals together is known as welding. Most of the time, the two metals are heated to extremely high temperatures. They melt together when they heat up. As the metals heat up, though, they begin to react with oxygen. In this reaction, a metal-oxygen combination is created. If the two metals have formed compounds, joining them becomes extremely difficult, but injecting argon into the welding atmosphere helps to strengthen the bond.
Healthcare Industry
- Argon is also used in the production of argon lasers and argon-dye lasers. A laser is a device that emits extremely strong light of a single color (frequency).
- Skin problems are treated using an argon laser. The laser beams a blue-green light onto the afflicted skin region. The laser’s energy is absorbed by hemoglobin and transformed to heat.
- Argon lasers are utilized in the treatment of diabetic retinal detachment and retinal phototherapy; they are also employed in surgery to fuse arteries and eliminate tumors.
- An argon-dye laser is used in eye surgery. The color of light produced by the laser can be adjusted with high precision
- Argon is also used to treat further problems, such as heart arrhythmias; alterations in the rhythm of the heartbeat.
- You may have read in the news about a dentist who utilized argon gas to eliminate bacteria on teeth to prevent cavities and gingivitis. Bacteria and fungi on teeth are killed by the gas by damaging their cell membranes, causing them to die. This aids in the prevention of bad breath and cavities caused by germs and fungus.
Food and Drink Industry
- Because of its inertness, argon can be found in the food and beverage industries.
- It is frequently used in wine containers because it is denser than air and sits above the liquid, protecting it from oxidation and souring.
- Argon is also used to preserve chips in packet .Other noble gases would be suitable for most of these purposes, but argon is by far the most affordable.
- Argon is cheap because it exists naturally in the air and is easily produced as a byproduct of cryogenic air separation in the creation of liquid oxygen and liquid nitrogen: the principal elements of air that are widely used in industry.
Document Preservation
One of the most exciting applications of argon gas is the preservation of historical documents. Because argon gas is inert, it can offer a protective environment. This protects them from degrading and being damaged during storage and exhibition.
Luxury Car Tyres
Argon gas is utilized in the tires of high-end automobiles. The gas shields the rubber from oxygen attack and ensures less tire noise at high speeds.
Scuba Diver
This noble gas can be utilized to insulate dry suits for cold-water diving. This is due to argon’s inertness and limited heat conductivity. It can also be used in its argox form, which is a common acronym for argon and oxygen-based scuba diving breathing gas. This use, however, is quite uncommon.
Detection of Neutrons
Neutron Scintillation Detection is a method for detecting high-energy neutron particles using argon gas. Because of their size and speed, as well as the fact that they frequently leave no mark when they contact a surface, these particles are extremely difficult to detect. Argon gas can detect them because neutrons hit with atoms in the gas, creating detectable gamma rays. This procedure aids scientists in their research into the characteristics of radioactive elements, nuclear weapons development, and nuclear manufacturing facilities.
Purification of Air
Because it absorbs oxygen and has a great capacity for eliminating other gases such as carbon dioxide and ozone, argon has been used to clean air. These gases can aggravate asthma episodes, bronchitis, and other respiratory issues.
Health Effects of Argon
Argon is a rare gas that is colorless and odorless and is widely utilized in industry to cleanse steel, fill light bulbs, and weld materials such as stainless steel, manganese, aluminum, and other specific metals.
- At atmospheric pressure, normal air contains 0.93% argon, which is non-toxic. However, when the quantity of argon in the air reaches 33% or more, it can induce asphyxiation.
- Long-term welding fume exposure may cause lung damage and a variety of cancers, including lung, throat, and urinary tract cancer.
- Gases such as argon and carbon dioxide transfer oxygen in the air and can induce hypoxia, particularly when welding in confined or enclosed spaces.
- Although long-term exposure to Argon gas under specific conditions can be harmful, it is not toxic in general.
Environmental Effects of Argon
Argon has no known record of any environmental impact till now. No adverse environmental consequences are expected. Argon gas is found in the environment naturally. And in well-ventilated environments, the gas will evaporate quickly.
The effects of argon on plants and animals are unknown at this time. It is unlikely to have any negative effects on aquatic life.
Argon does not contain any ozone depleting chemicals and is not listed as a marine pollutant.
Fun Facts About Argon
- Argon-39 is used much like carbon-14 to date water and ice samples.
- Worldwide over 771 million tons of argon gas are produced annually.
- Double paned windows use argon between the panes to act as an insulator.
- NASA probes have discovered argon in Mercury’s atmosphere and on Saturn’s moon Titan.
- Even though it is not poisonous, it can still cause suffocation because it displaces air due to its high density.
- If we move out of Earth, Argon-36 is the most abundant isotope. This isotope (that is Argon-36) is produced in stars.
- The original United States Declaration of Independence is stored in a case filled with argon gas to protect it from decay.
- Rayleigh, in his Nobel Prize speech, said that no one should consider Argon as a rare gas because the room (in which the ceremony was taking place) can very easily contain more Argon (by weight) than what a single person can carry.
- Archeologists who discovered the presence of Homo erectus 1.8 million years ago examined the volcanic pumice found inside the skulls they discovered. The scientists were able to acquire precision dating by analyzing the volcanic pumice with Potassium-Argon followed by Argon-Argon dating.
Handling and Storage of Argon Gas Cylinder
- Cylinders must not be dragged, dropped, slid, or rolled. The uncontrolled discharge of a pressurized gas may result in bodily damage. Use an appropriate hand truck to transfer the cylinder.
- Cylinders should be stored at temperatures below 45°C in a safe location, upright and restrained to prevent them from dropping.
- Cylinders should also be housed in a dry, well-ventilated space made of non-combustible materials with a solid level surface (ideally concrete), away from high traffic areas and emergency exits.
Watch out this video for all important information about Argon.
References
- John H. Wolfenden, The Noble Gases and the Periodic Table: Telling it like it was., J. Chem. Educ., 1969, 46 (9), p569.
- https://education.jlab.org/itselemental/ele018.html
- Brown, T. L.; Bursten, B. E.; LeMay, H. E. (2006). J. Challice; N. Folchetti (eds.). Chemistry: The Central Science (10th ed.). Pearson Education ISBN 978-0-13-109686-8.
- https://www.rsc.org/periodic-table/element/18/argon
- William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1-4398-5511-0.
- Mary Elvira Weeks, The Discovery of the Elements. XVIII. The Inert Gases., J. Chem. Educ., 1932, 9 (12), p2065.
- https://www.lenntech.com/periodic/elements/ar.htm#ixzz7znh5bOtw
- https://www.chemicool.com/elements/argon.html
- Khriachtchev, Leonid; Pettersson, Mika; Runeberg, Nino; Lundell, Jan; et al. (2000). “A stable argon compound”. Nature. 406 (6798): 874–876. Bibcode:2000Natur.406..874K. doi:10.1038/35022551. PMID 10972285. S2CID 4382128
- Vivi Ringnes, Origin of the Names of Chemical Elements, J. Chem. Educ., 1989, 66 (9), p731.
- Lord Rayleigh, The Density of Gases in the Air and the Discovery of Argon, Nobel Lecture, December 12, 1904.
- Hiebert, E. N. (1963). “In Noble-Gas Compounds”. In Hyman, H. H. (ed.). Historical Remarks on the Discovery of Argon: The First Noble Gas. University of Chicago Press.

