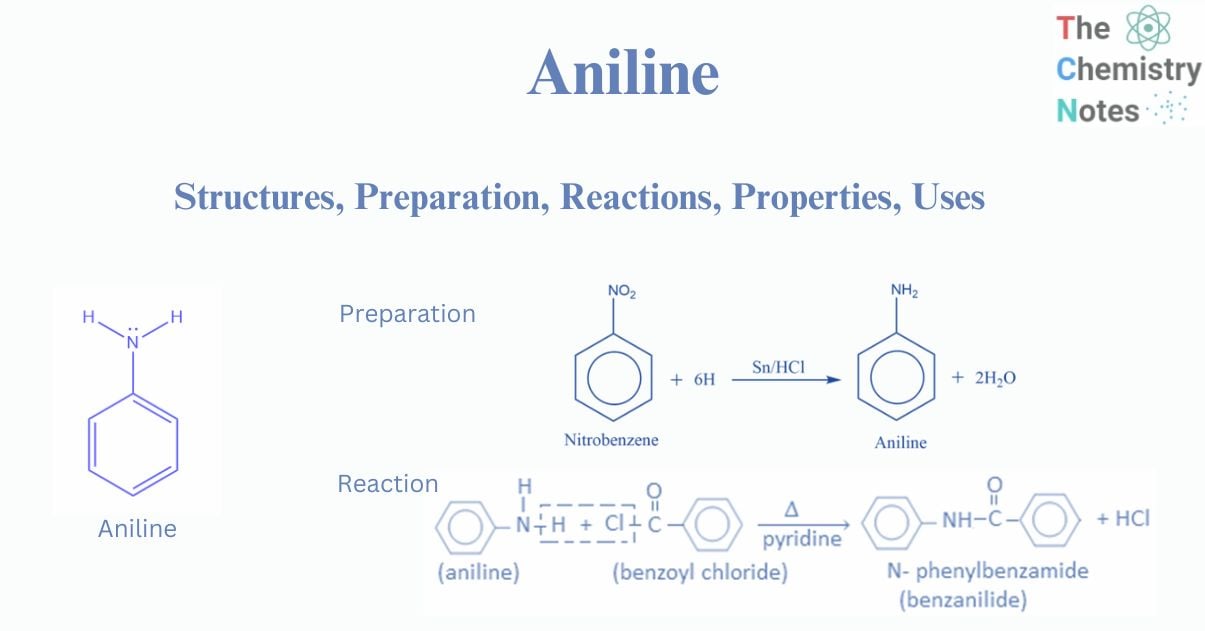
Aniline is an aromatic derivative in which a hydrogen atom of the benzene ring has been replaced by an amino group -NH2. Aniline is also known as aminobenzene or phenylamine. It has a chemical formula of C6H7N or C6H5NH2 that contains Six carbon (C) atoms, Seven hydrogens (H) atoms, and One nitrogen (N) atom. Aniline is categorized as an aromatic amine because it is likewise an amine and has an amino group in its structure.
The destructive distillation of indigo yielded aniline for the first time in 1826. It derives its name from the indigo-producing plant Indigofera anil (Indigofera suffruticosa). Aniline is the most basic aromatic amine. Anilines are a key industrial essential chemical. It has the odor of rotten fish, as do other volatile amines. It burns easily. It has a smoky flame, which is typical of aromatic chemicals.
Aniline is a viscous yellowish-to-brownish liquid with a musty, fishy odor. melting point of -6°C; boiling temperature of 184°C and slightly denser than water. Aniline vapors have a higher density than air. Pure aniline is an extremely toxic, colorless, oily chemical with a pleasant odor.
Aniline is a benzene derivative with a high electron density. As a result, aniline undergoes fast electrophilic aromatic substitution reactions. It is also susceptible to oxidation.
Structure of aniline
The molecule of aniline has a pyramidal shape with a nitrogen hybridization that resides between sp3 and sp2. As a result, the nitrogen lone pair is in an spx hybrid orbital with a high p character. Because of the conjugation of the lone pair with the aryl substituent, the amino group in aniline is flatter than that of an aliphatic amine.
The geometry demonstrates an arrangement between two competing factors: stabilization of the N lone pair in an orbital with a significant s character favors pyramidalization, i.e., orbitals with an s character have lower energy. Delocalization of the N lone pair into the aryl ring favors planarity, i.e., a lone pair in a pure p orbital has the best overlap with the benzene ring pie system orbitals.
Preparation of Aniline
Laboratory preparation
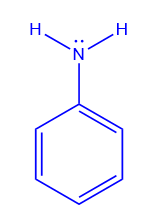
Aniline is produced in the laboratory by reducing nitrobenzene with tin and concentrated hydrochloric acid.
From the reduction of nitrobenzene with iron and dil.HCl
Aniline is commercially produced by reducing nitrobenzene with iron and dil. HCl.
4 C6H5NO2 + 9 Fe + 4 H2O → 4 C6H5NH2 + 3Fe3O4
From the reaction of chlorobenzene with ammonia
Chlorobenzene reacts with ammonia in the presence of CuCl2 as a catalyst to give aniline.
C6H5Cl + 2 NH3 + CuCl2 → C6H5NH2 + NH4Cl
From benzamide
When amides are heated with bromine and caustic potash, the amide (-CONH2) group is converted into the amine (-NH2) group. This is known as Hoffman’s degradation reaction. This process produces aniline from benzamide.
C6H5CONH2 + Br2 + 4 KOH → C6H5NH2 + K2CO3 + 2 KBr + 2 H2O
Physical properties of aniline
- Aniline has a boiling point of around 184 oC and a melting point of approximately 6 oC.
- It has a characteristic odor.
- Density of aniline is 1.002 at 20 oC .
- It is sparingly soluble in water. It is readily soluble in a wide range of solvents, including alcohol and ether.
- It becomes pale yellow and darkens When exposed to air.
- It is a weak base that reacts with strong acids to generate anilinium ion C6H5NH3+.
- When the substance is inhaled through the air or absorbed through the skin, it forms nitrogen oxides that cause harmful effects on the environment.
- Aniline is steam volatile and highly toxic.
Reactions of aniline
Reactions involving -the NH2 group
Basic nature
- Because of the presence of an electron lone pair on the nitrogen atom, it is basic in nature.
- It is a weaker base than ammonia and aliphatic amines. This is due to the fact that the amines group in aniline is linked to the phenol group, which is negative. The -I effect of the phenol group and the + M effect of the amines group diminish the availability of a lone electron pair on nitrogen.
- The addition of an electron-donating group raises the basicity of the benzene ring. Based on this, ortho meta and para Toluidine are stronger bases than aniline.
- Because it is basic in nature, it combines with acid to form salt. Eg.

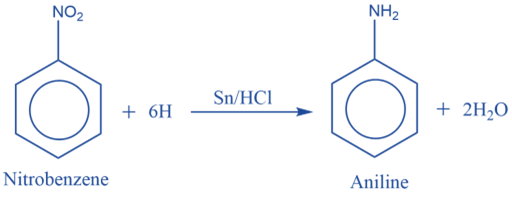
Reaction with an alkyl halide
Alkylation is the process of replacing the H-atom of an amino group with an alkyl group (-R).
The reaction of aniline with an alkyl halide produces secondary, tertiary, and quaternary ammonium compounds.
Reaction with benzaldehyde
Aniline reacts with benzaldehyde to give benzylidene aniline
C6H5 -NH2 + C6H5CHO → C6H5 — N = CH — C6H5 + H2O
Acylation of aniline
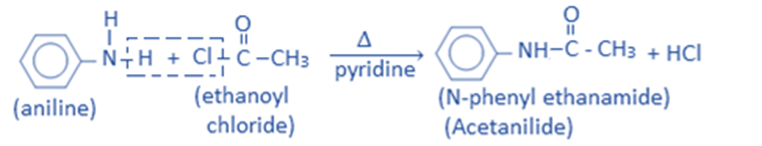
The reaction of aniline with an acid chloride or acid anhydride produces corresponding amides called anilides. For example, In the presence of pyridine, aniline reacts with ethanoyl chloride to give N-phenyl ethanamide ie., acetanilide.
Carbylamine reaction
The reaction of aniline with the chloroform and alc. Koh produces phenyl isocyanide (carbylamine), which has an offensive smell.
C6H5 -NH2 + CHCl3 + 3 KOH → C6H5 — NC + 3H2O + 3 KCl
Benzoylation
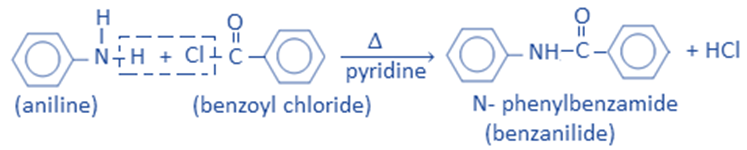
Benzoylation is the process of replacing the H-atom of an amino group with a benzoyl group (C6H5CO-). Aniline reacts with the benzoyl chloride to give benzanilide.
Reaction with nitrous acid
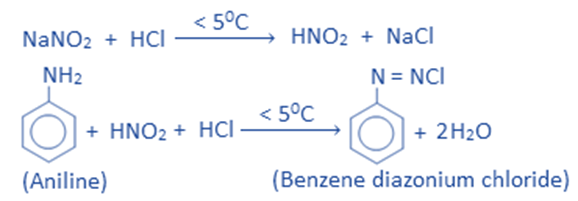
In the presence of hydrochloric acid, aniline reacts with nitrous acid to produce benzene diazonium chloride. The action of NaNO2 and HCl produces the nitrous acid required for this process. Since benzene di diazonium chloride decomposes at higher temperatures, this reaction is usually carried out at low temperatures (0-5°C). The -NH2 group is transformed into the -N group in this reaction. This is known as the diazotization reaction.
The existing benzene diazonium chloride — N = N — Cl group can be easily changed into different groups. As a result, this reaction is used to produce a wide range of chemicals, including aniline, benzene, chlorobenzene, phenol, and others. In alkaline solutions, benzene diazonium chloride reacts with beta-naphthyl and a few other chemicals.
Reactions involving benzene ring
Electrophilic substitution reactions
The electrophilic substitution reaction occurs when an electrophile substitutes another electrophile in an organic molecule. Halogenation, nitration, and sulphonation are common electrophilic processes for anilines. The functional group (-NH2) connected to aniline is an electron-donating group, making it highly active for the electrophilic substitution process.
Because of its multiple resonant configurations, the benzene ring has more electrons or negative charge in the ortho- and para- positions than in the meta- position. As a result, anilines are o- and p-directed in the electrophilic substitution process.
Bromination reaction
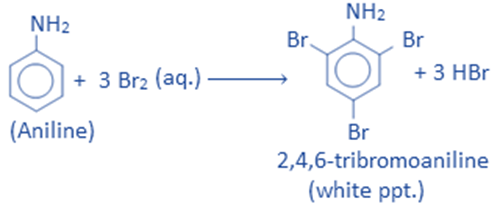
When aniline interacts with liquid bromine, it produces 2,4,6-tribromoaniline, a white powder. Benzene does not react with liquid bromine, whereas aniline does. This reaction demonstrates that aniline has a higher electron density in the benzene ring than benzene.
Suphonation reaction
Aniline and sulphuric acid react quickly to make anilinium hydrogen sulfate, which when heated, yields sulphanilic acid.
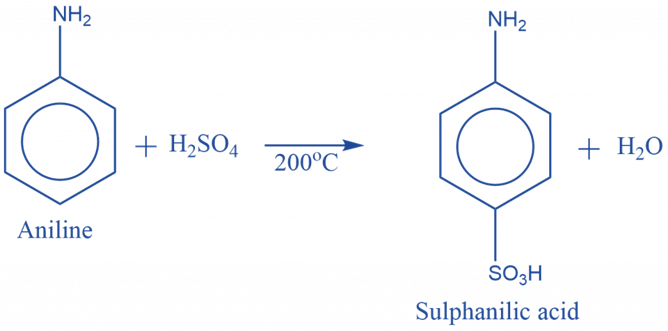
Nitration reaction
Aniline cannot be nitrated directly because, in addition to being a nitrating agent, conc. HNO3 is also an oxidizing agent that may oxidize amino groups. As a result, before nitration, the amino group is protected by acetylation. The nitro group can then be placed into the ring at the ortho and para positions. Nitro aniline is produced by hydrolysis of nitrated-acetylated aniline. The para product is obtained as the major product.
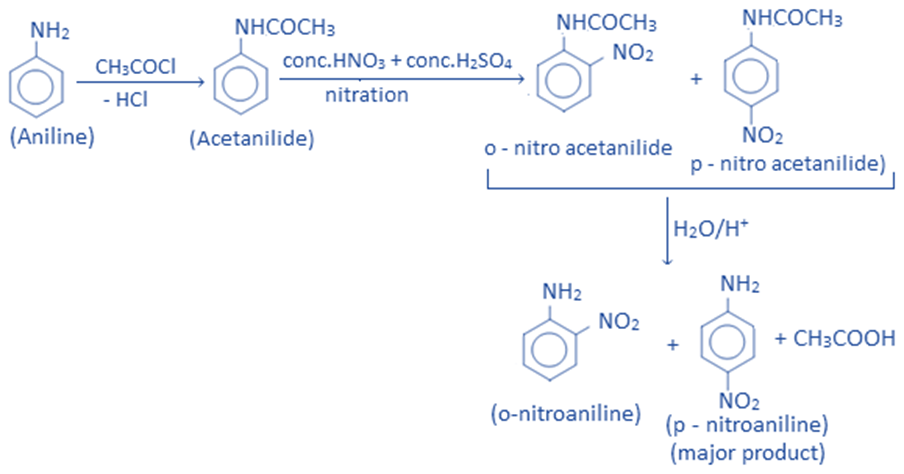
Uses of aniline
- It is used as the starting material for the preparation of various chemical compounds.
- This chemical is used in the production of drugs such as paracetamol, acetaminophen, and Tylenol.
- In agriculture, this chemical is employed as a pesticide and fungicide.
- It is used in the production of polyurethane, which is then utilized in the production of plastics.
- It is also utilized in the rubber industry in the process of vulcanization.
- Anilines are used in products such as automobile tires, gloves, balloons, and so on.
- It is also utilized as a coloring agent in the production of clothing such as jeans.
- It determines the aromaticity of oil products.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- https://www.britannica.com/science/aniline.
- https://byjus.com/chemistry/anilines/.
- https://www.chemistryscl.com/advancedlevel/organic/aniline/main.html.
- https://www.vedantu.com/chemistry/anilines
