A functional group is a specific group of atoms within a molecule that is responsible for a molecule’s characteristic. One or more functional groups are found in many biologically active compounds. The various functional groups are hydroxyl, methyl, carboxyl, carbonyl, and amino functional group.
What are Amino Groups?
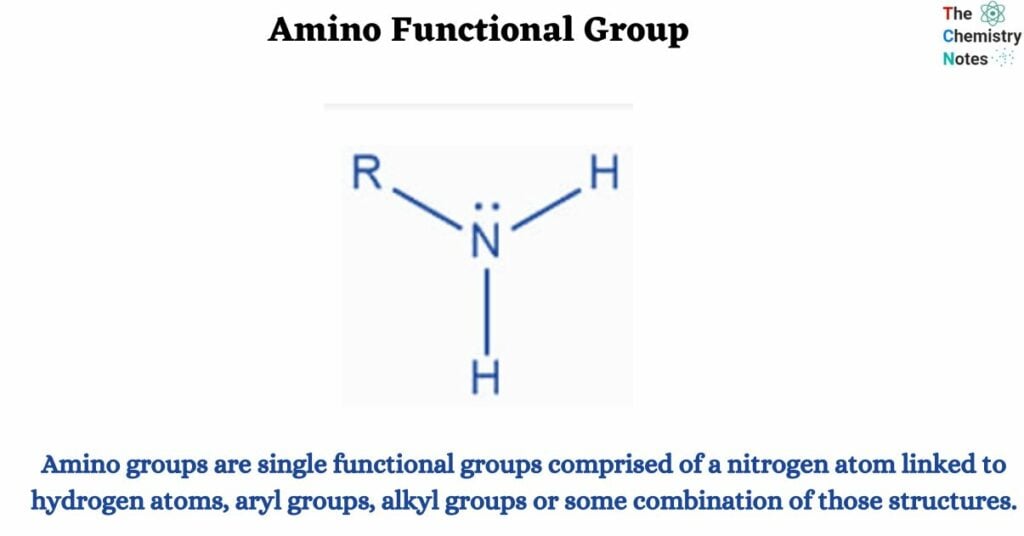
Amino groups are single functional groups comprised of a nitrogen atom linked to hydrogen atoms, aryl groups, alkyl groups or some combination of those structures. If an amino group is attached to an organic compound (compounds containing carbon) it is referred to as an amine.
The most notable amines are amino acids. An examination of the composition of amino groups, and the way they form structures such as amino acids, will help make amino groups clearer.
What is an Amino Functional Group?
An amino group is made up of a nitrogen atom linked to two hydrogen atoms. An amine is a molecule that includes an amino functional group.
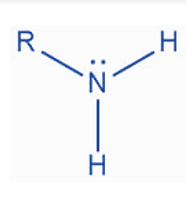
Amino groups are sometimes known as amine groups. Amino is a subclass of amine. Amine is any nitrogen-containing group with a lone pair. The amino group is particularly nitrogen with a lone pair and at least two hydrogens bound to it. A primary amine is an amino group. The term primary refers to having only one non-hydrogen bond.
The nitrogen atom is responsible for the amino group’s fundamental characteristic. This atom provides its lone pair of electrons from the valency shell when needed, enhancing the amino group’s reactivity and interaction with other molecules.
Physical Properties of Amino Functional Group
- Amino functional groups can form hydrogen bonds. As a result, their boiling temperatures are higher than those of the comparable phosphines but lower than those of the corresponding alcohols, which hydrogen bond to a greater extent.
- They can also be dissolved in water. However, the solubility diminishes with increasing carbon atoms due to the compound’s increased hydrophobicity as chain length grows.
- Aliphatic amines, or amines linked to an alkyl chain, are soluble in organic polar solvents.
- Aromatic amines, which participate in a conjugated ring, contribute their lone pair of electrons into the benzene ring, reducing their capacity to engage in hydrogen bonding.
Amino Group Reactions
Because of the lone pair on the nitrogen atom, amino groups respond quickly. They are also more basic than many alcoholic beverages. An activating group is an NH2 group.
To produce R-NH3+, an amino group can take H+.
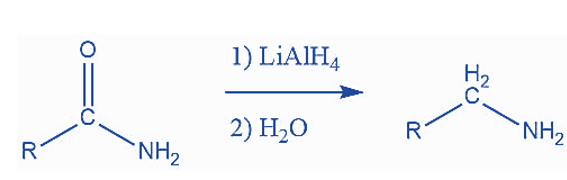
Reactions Forming an Amino Group
When nitriles and amides are reduced, amino groups are formed. This reaction is most typically performed with LiAlH4. Here’s an example of this reaction:
Nitration of an aromatic molecule followed by reduction is an excellent method for preparing amino groups on an aryl group. This sort of reaction is catalyzed by platinum or other metal catalysts such as zinc or iron.
Test for Amino Functional Groups
The following tests are performed to determine the presence of an amino group in an organic molecule.
Solubility
Amines are organic molecules that are basic in nature, therefore they dissolve in mineral acids such as hydrochloric acid. However, this is not a confirmatory test for amines.
The chemical reaction is illustrated below:
C6H5NH2 + HCl → C6H5NH3+Cl–
It might be an amine if it is soluble in mineral acid.
Litmus test
Amines are the most fundamental elements in nature. As a result, it turns red litmus paper blue. This test is also not an amine confirmation test.
The chemical reaction is illustrated below.
CH3-CH2-NH2 + H2O → CH3-CH2-NH3+ + OH–
The shift in color of red litmus paper indicates that the provided organic molecule is a base.
Carbylamines Test
This test is also known as the isocyanide test because isocyanide is generated when amines are treated with chloroform in the presence of alkali. This test is positive for both aliphatic and aromatic amines. This test is not performed on secondary or tertiary amines.
The chemical reaction is illustrated below.
R-NH2 + 3KOH + CHCl3 → RNC(isocyanide) + 3KCl + 3H2O
An unpleasant odor indicates the presence of primary amine.
Nitrous Acid Test
This test can distinguish between primary, secondary, and tertiary amines. An aromatic primary amine combines with nitrous acid to generate a diazonium salt, and the resulting diazonium compounds decompose at higher temperatures.
C6H5NH2 + HNO2 → C6H5-N≡N+Cl–(diazonium compound)
When primary aliphatic amines react with nitrous acid, they produce nitrogen gas, which appears as bubbles.
R-NH2 + HONO → R-OH + H2O + N2↑
Secondary amines combine with nitrous acid to generate nitrosamine, a yellow oily compound.
R2-NH + HONO → R2N-NO + H2O
Tertiary amines create soluble nitrite salts when they react with nitrous acid.
R3N + HONO → R3NH+NO2–
Nitrosamines are extremely carcinogenic chemicals.
Importance of Amino Functional Groups in Medicine
Amino groups have a lot of benefits in medical science. Amino groups are found in many helpful drugs. Penicillin, for example, has bacterial-busting strength due to a unique four-membered beta-lactam ring attached to a five-membered thiazolidine ring containing an amino group. Metformin, a type 2 diabetic treatment, also contains an amino group.
Gene treatment: Amino groups are also gaining attention in gene treatment. Polymeric nanoparticles that encapsulate and carry gene material frequently have amino groups in their structure to aid in DNA condensation and gene transfection rates. Finally, the amino group, a seemingly basic substructure, plays an important role in our daily lives, demonstrating the effect and necessity of biochemistry. It continues to have an influence on many aspects of human existence despite being vital to various businesses and health science professions.
Amino Group in Biochemistry
Protein Synthesis: Proteins are the building blocks of life, yet they couldn’t exist without amino groups! The peptide bond between amino acids is formed by the interaction of one amino acid’s carboxyl group with the amino group of another, releasing water in the process. Proteins’ basic structure is formed by this bond, which contributes greatly to their ultimate (tertiary) structure.
Peptide bond: A covalent connection created by the condensation process of one amino acid’s carboxyl group and the amino group of the other, releasing a water molecule.
pH regulation: Because amino groups are basic, they play an important function in maintaining the delicate pH equilibrium required by living organisms. They may absorb and donate protons, assisting in the maintenance of appropriate pH levels in cells and tissues. Neurotransmission: Amino groups are found in neurotransmitters such as dopamine and norepinephrine, which are both important in mood regulation and rewarding behavior in the brain.
References
- https://uen.pressbooks.pub/introductorychemistry/chapter/functional-groups-names-properties-and-reactions/
- https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A%3A_Introductory_Biology_(Britt)/01%3A_Readings/1.09%3A_Functional_Groups#:~:text=The%20amino%20group%20consists%20of,group%20displaying%20some%20polar%20character.
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/amino-group/
- https://byjus.com/chemistry/test-for-amino-groups/
- https://chemistrytalk.org/amino-functional-group/
