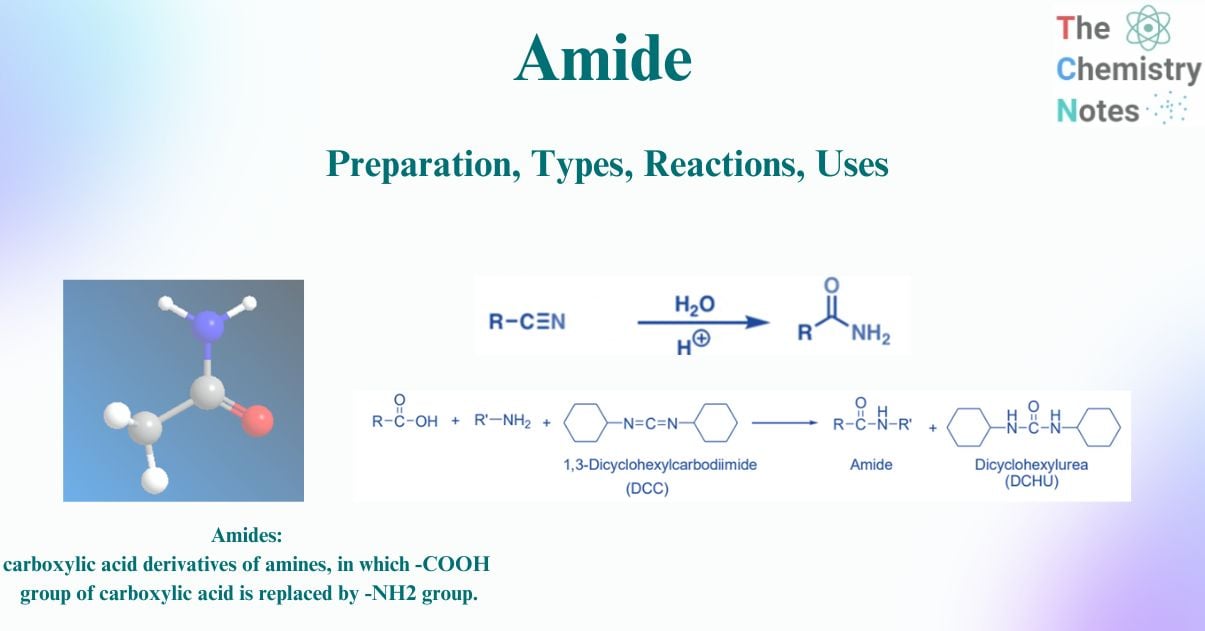
Amides are carboxylic acid derivatives of amines. A carboxylic acid has the -COOH group, and in amide, the -OH group of the carboxylic acid is substituted by the -NH2 (amino) group, resulting in the -CONH2 group. They are therefore compounds with the functional group RCONH2 or RCONR2, where R can be an organic group (alkyl or phenyl) or hydrogen (H).
Amides are compounds containing a carbonyl group coupled to an amine in addition to a hydrocarbon group (or hydrogen atom).
As a result, they are either of two types of nitrogen-containing molecules that are associated with ammonia and amines. Acetamide, also known as ethanamide (CH3CONH2), and dimethylformamide HCON(CH3)2 are commercially important amides that are utilized as solvents, sulfa medicines, and nylons.
Types of amides
On the basis of the number of substituents (non-hydrogen-like groups) connected to the nitrogen of the amide group amides are classified into three groups. They are:
Primary amide:
In primary (1°) amide nitrogen is attached to a single carbon.
HCONH2 Methanamide (Formamide)
CH3CONH2 Ethanamide (Acetamide)
Secondary amide:
a secondary (2°) amide is a compound with a nitrogen attached to two carbons. In these, alkyl group acts that are linked to nitrogen are named substituents. The letter N is used to denote that they are attached to a nitrogen atom.
CH3CH2CONHCH3 (N-methylpropanamide)
CH3CH2CONHC6H5 (N-phenylpropanamide)
Tertiary amide
A tertiary (3°) amide consist of a nitrogen attached to three carbons. They are designated in the same way as secondary amides but with two substituents on nitrogen atoms instead of one.
HCON(CH3)2 (N, N-dimethylformamide or N, N-dimethylmethanamide)
CH3CON(CH3)C2H5 (N-ethyl-N- methylethanamide)
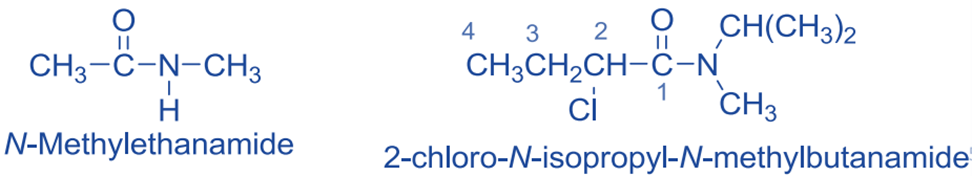
Nomenclature
An amide is named for the fact that it is a mixture of carboxylic acid and ammonia or an amine.
Primary amides are thus named by replacing the suffix -oic acid in the IUPAC name of the amide’s parent carboxylic acid with the suffix -amide. The root name is based on the longest chain that includes the amide group’s carbonyl group. As an example:
CH3COOH → CH3CONH2
Acetic acid Acetamide
The alkyl groups on nitrogen are treated as substituents in the nomenclature of secondary and tertiary amides, and their position is given by the prefix N- to distinguish them from any substituents attached to the parent chain of the parent carboxylic acid. If more than one substituent is attached to nitrogen, they are listed alphabetically after the amide name.
Preparation of amides
By heating Ammonium Carboxylate
The carboxylic acid is first transformed into an ammonium salt, which is then heated to produce an amide.
The ammonium salt is created by adding solid ammonium carbonate to an excess of acid.
Ammonium ethanoate, for example, is formed by the reaction of ammonium carbonate to an excess of ethanoic acid.
2 CH3COOH + (NH4)2CO3 → 2 CH3COONH4 + H2O + CO
When the reaction is finished, the mixture is heated, and the ammonium salt dehydrates, resulting in ethanamide.
CH3COONH4 → CH3CONH2 + H2O
The extra ethanoic acid prevents the ammonium salt from dissociating before it dehydrates.
From acyl chloride
Acyl chloride is frequently referred to as acid chloride (RCOCl). The chlorine atom is easily replaced by another atom or group, such as the -NH2 group, to make an amide. A mixture of solid ammonium chloride and ethanamide undergoes an intense reaction that produces a large amount of white smoke. Some of the mixture is dissolved in water and remains colorless.
Reactions involved:
i. Formation of ethanamide and hydrogen gas
CH3COCl + NH3 → CH3CONH2 + HCl
ii. Formation of ammonium chloride
The generated hydrogen chloride then interacts with excess ammonia to form ammonium chloride.
NH3 + HCl → NH4Cl
Overall reaction:
CH3COCl + NH3 → CH3CONH2 + NH4Cl
From acid anhydride
The reaction of acid anhydride with ammonia produces amide. if ethanoic anhydride is added to a concentrated ammonia solution, ethanamide and ammonium ethanoate are formed.
(CH3CO)2O + 2NH3 → CH3CONH2 + CH3COONH4
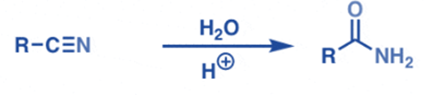
From partial hydrolysis of nitriles
Under acidic or basic circumstances, nitriles can be hydrolyzed to produce primary amides.
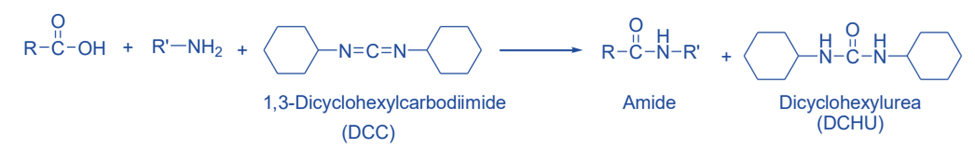
From DCC (N, N-Dicyclohexylcarbodiimide)
In the presence of DCC (N, N-Dicyclohexylcarbodiimide), primary and secondary amines react with carboxylic acids to form amides. DCC acts to activate the carboxyl group of the carboxylic acid, facilitating coupling to the amino group.
Physical properties of amides
- Formamide is a liquid, whereas all other amides are colorless and crystalline at room temperature.
- Lower aliphatic amides with up to six carbons are water-soluble. As the size of the alkyl groups increases, so does their solubility.
- Because primary and secondary amides can establish hydrogen bonds, they have high boiling and melting points. As tertiary amides cannot hydrogen bind, their boiling temperatures are lower than those of amides of equivalent size.
Chemical properties of amides
- Amides are the most stable carboxylic acid derivatives and, as a result, the least reactive carboxylic acid derivatives. The reduced reactivity of amides is due to the fact that they exist in the imidate state rather than as a true amide. The electrophilicity of the carbonyl’s carbon is greatly reduced by imidate production. As a result, amides have fewer reactions.
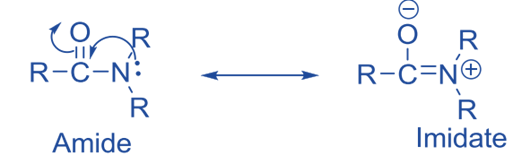
- of all the acid derivatives, amides are the least reactive to nucleophilic acyl substitution reactions.
- Amides are much less basic than amines due to the delocalization of the lone pair of nitrogen.
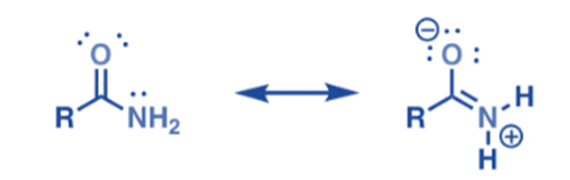
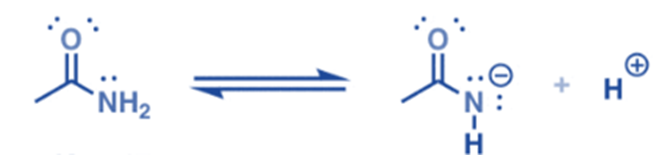
- Amides can behave as behave both as weak bases and weak acids, due to resonance.
Acid character: 2 CH3CONH2 + HgO → (CH3CONH2)2Hg + H2O
Base character: CH3CONH2 + HCl → CH3COCl + NH3
- Since the lone pair of the conjugate base is stabilized through resonance delocalization, N—H bonds of amides are considerably more acidic than N—H bonds of amine.
- Amides can be hydrolyzed by boiling with water, acids, or alkalis.
- A more subtle feature of amides is that they typically have limited rotation around the C-N bond. Because the resonance form where the C-N bond contributes so significantly to the resonance hybrid, the C-N bond can be thought of as having a “partial double-bond character.”
Reactions of amides
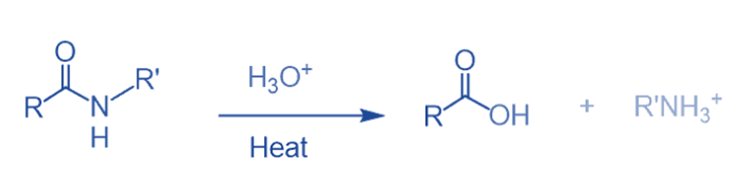
Hydrolysis
Carboxylic acid is produced during the hydrolysis of the amide with strong acids or bases under extreme reaction conditions. The hydrolysis of amides is significantly more difficult than that of esters.
Reaction with alcohol
Amides react with alcohol to give esters. It requires high heat and strong acids, just like hydrolysis. As a result, this approach has minimal practical utility.
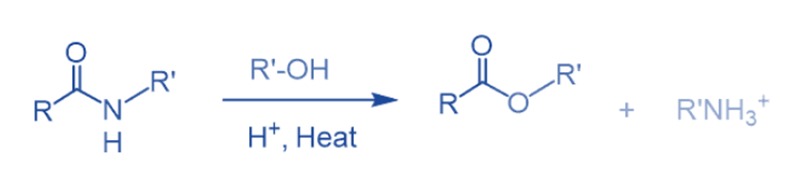
Esters can be changed to amides because the ester’s OR- the group is a better-leaving group than an amine’s conjugate base. So, by reacting a substantial excess of alcohol with amide, this reaction can be driven in the other way.
Reduction
Amides react with a strong reducing agent i.e. lithium aluminum hydride (LiAlH4) to give primary amine.
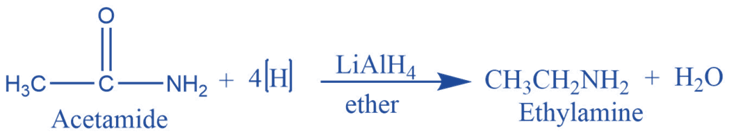
The reaction provides a route for the synthesis of amines of various classes from amides and, eventually, carboxylic acids.
Hofmann’s degradation of amides
A primary amine is formed by heating the amide with bromine and a concentrated aqueous solution of NaOH solution. It is an effective method to prepare primary amine in the laboratory. This is also known as Hofmann’s rearrangement reaction. The final product has one less carbon than the original amide.

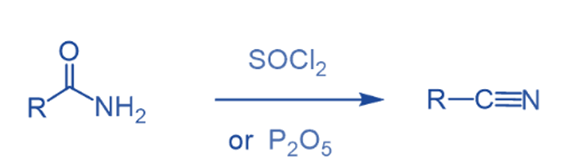
Reaction with a dehydrating agent
Amides on heating with strong dehydrating agents like P2O5, SOCl2, or POCl3 produce nitriles. Nitriles are of great importance in organic synthesis because, unlike amides, can undergo a number of essential reactions.
Reaction with nitrous acid
Amides, in reaction with nitrous acid, give carboxylic acids and nitrogen gas.
Nitrous acid is produced in situ via the interaction of sodium nitrite and hydrochloric acid.
NaNO2 + HCl → NaCl + HNO2 (HONO)
CH3CONH2 + HNO2 → CH3COOH + N2 + H2O
Uses of amides
- Amides are utilized in the synthesis of primary amines.
- Dimethylformamide and dimethyl acetamide are employed for polar and non-polar chemicals.
- Amides are widely utilized in the manufacturing of plastic, rubber, paper, color in crayons, pencils, and ink, as well as water and sewage treatment.
- Polyacrylamide is utilized in the treatment of sewage and drinking water.
- Acetamide acts as an antidote when fluoroacetamide targets cardiac muscles, as this poison causes degeneration and necrosis. Acetamide is the only molecule that can counteract its effects.
- Benzamide medications are employed to stimulate acetylcholine receptors in smooth muscles while blocking dopamine and serotonin receptors.
- Proteins include amides, which bind amino acids together.
- Acrylamide is a chemical that is used in the beauty industry to make soap, hair products, and preshave lotion.
- These are found in complex structural proteins like keratin as well as essential hormones like insulin. Amide bonds are peptide and protein peptide bonds.
References
- March J. (1977). Advanced organic chemistry : reactions mechanisms and structure (2d ed.). McGraw-Hill.
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- https://www.britannica.com/science/amide.
- https://www.chemguide.co.uk/organicprops/amides/preparation.html.
- https://www.masterorganicchemistry.com/2018/02/28/amides-properties-synthesis-and-nomenclature/.
- https://byjus.com/chemistry/amido-and-amide/.
