Aluminium is a noncorrosive lightweight silvery-white metal. It is included in the boron group (Group III a) in the periodic table. Aluminium is the most abundant metallic element on earth accounting for about 8% of the earth’s crust. It can be found in clay, slate, and various silicate rocks. It can only be discovered when everything is blended.
Interesting Science Videos
Ores of aluminium
Due to its chemical reactivity aluminium does not occur as a free element on the surface of the earth. It is seen as different ores. Bauxite ore is the most commonly found form of aluminium.
- Bauxite Al2O3. 2H2O
- Corundum Al2O3
- Cryolite Na3AlF3
- Alunite K.2SO4.Al2(SO4)3.4Al(OH)3
- Kaolinite Al2O3 2SiO2·2H2O
- Nepheline Na3KAl4Si4O16
Production of aluminium
- France 1855: H. Sainte-Clarie Deville first reduced aluminium chloride with sodium.
- Austria 1888: Karl Josef Bayer first discovered the Bayer process (digesting crushed bauxite in strong sodium hydroxide solution at temperature up to 240oC.
- Germany: Hall-Heroult introduced the Hall-Heroult process by dissolving the alumina in molten cryolite (Na3AlF6)
- United States: Alcoa started a chloride-based smelting process using alumina combined with chloride
Extraction of aluminium
Metallurgy is the science of extracting pure metals from their ores using cost-effective methods. Aluminium metallurgy, or the extraction of aluminium from its ore, involves a variety of methods.
- Dressing or Concentration of the Bauxite ore by Hall’s method
- Electrolysis by Hall – Heroult Method
Dressing or Concentration of the Bauxite ore by Hall’s method
The dressing is the process of extracting a metal from an ore in which gangue is removed and the ore is prepared for smelting, refining, or other processes.
Ferric oxide and silica are normally present as impurities in bauxite. Bauxite ore is dressed by crushing and pulverizing. Magnetic separation is used to remove ferric oxide impurities from bauxite. Hall’s method is used to concentrate the ore.
Charles Martin Hall, an American chemist, invented the hall method of concentrating bauxite in 1886. This is why the method is known as Hall’s method. It takes place by the following method:
Conversion of bauxite into sodium aluminate:
The ore is fused to red heat with sodium carbonate, and sodium aluminate is formed.
Al2O3.2H2O + Na2CO3 + heat → 2NaAlO2 +2H2O +CO2
2. Formation of sodium hydroxide from sodium aluminate:
2NaAlO2 +3H2O +CO2 → Na2CO3 + 2Al(OH)3
3. Conversion of sodium aluminate to pure alumina
At 1100℃ sodium hydroxide is converted to pure alumina.
2Al(OH)3 → Al2O3 + 3H2O
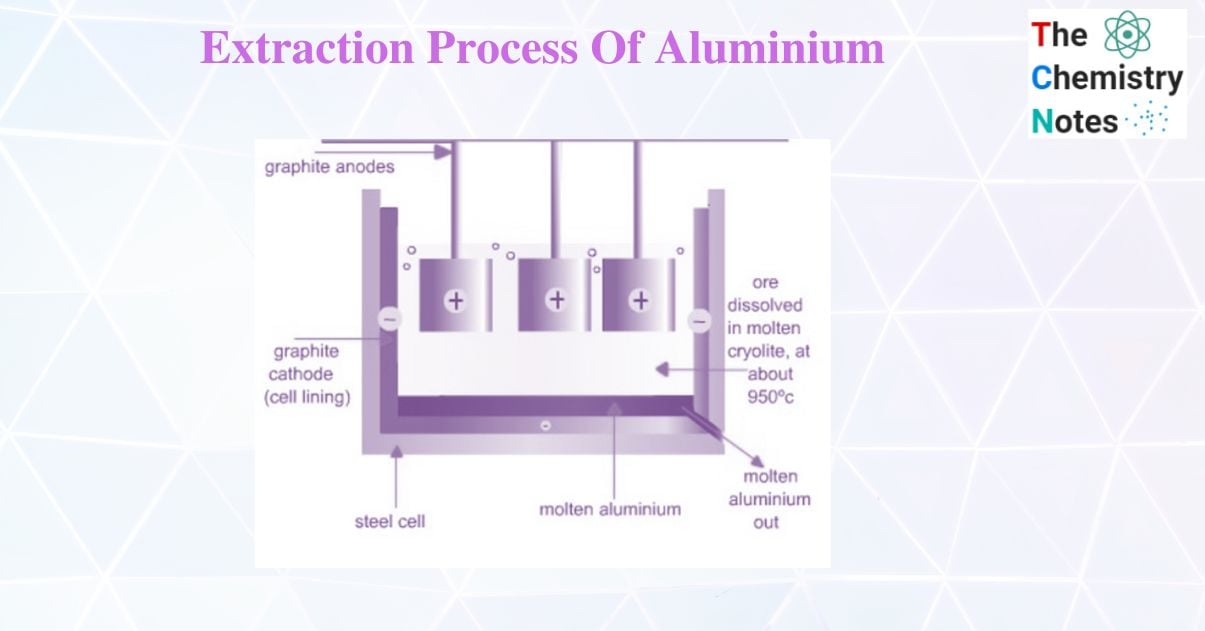
Extraction of aluminium by Hall – Heroult Method
Hall – Heroult Method process is widely used in the extraction of aluminium. In Hall – Heroult method pure bauxite (Al2O3) is mixed with cryolite Na3AlF6. The melting point of cryolite is 1000oc while that of bauxite is 2045oC. So, the cryolite acts as an impurity and helps in lowering the temperature of electrolysis. A steel vessel with the lining of carbon and graphite rod is used for electrolysis.
The carbon lining acts as a cathode and graphite acts as the anode. On electrolysis, Al and O2 are formed. Al is discharged at the cathode and sinks because they are heavier than cryolite solution. Therefore, the molten aluminium collects at the bottom of the cell and is tapped off periodically.
Oxygen is formed at the anode. This reacts with the carbon of the anode to form oxides of carbon at high temperatures. As a result, the anodes are gradually burnt away and must be replaced from time to time.
Functions of cryolite
- It increases the electrical conductivity of the cell as Al2O3 is a poor conductor.
- Cryolite acts as an impurity and lowers the melting point of the mixture to about 950oC.
Extraction of aluminium by Bayers process
In this process, aluminium ore is treated with concentrated sodium hydroxide to produce soluble aluminium silicate that can be filtrated. When the filtrate is heated with water, it produces aluminum hydroxide. Alumina is formed when aluminium hydroxide is strongly heated with water.
for more details use this link:
https://www.embibe.com/exams/extraction-of-aluminium/
Properties of aluminium
- Aluminium is a silver-white metal with a melting point of 660.323°C, and a boiling point of 2519°C.
- It is the lightest metal having a specific weight of 2.7 g/cm3, which is one-third that of steel.
- It is a good conductor of heat and electricity.
- It has high strength.
- It is non-magnetic and non-sparking.
- It is highly resistant to corrosion.
- It is non-toxic.
- It can be easily recycled
Uses of aluminium
- Aluminium foil is used for food packing.
- Due to its lightweight it is used in various applications in the field of transportation and, construction.
- It is also used for making customer products like cosmetics, and antiperspirants.
- It is a soft metal and can be moulded into any shape. So, it is used in the production of storage cans, bottles etc.
- Due to its high conductivity, aluminium is used to make electrical wires
- Finely divided aluminium is used for making aluminium paints.
- Al (OH)3 is used as an antiacid treatment for indigestion.
- It is also used in mirrors and telescope mirrors.
- Aluminium is used for making cooking utensils.
References
- https://byjus.com/chemistry/occurrence-and-extraction-of-aluminium/
- https://byjus.com/chemistry/chemical-properties-of-aluminium/
- https://www.embibe.com/exams/extraction-of-aluminium/
- https://www.slideshare.net/DanielCuevas33/aluminium-and-its-alloys-96585581
- https://www.vedantu.com/chemistry/aluminium-ore-extraction-of-aluminium
- https://www.slideshare.net/kaiwan1996/aluminium-51474084
- https://www.tms.org/pubs/books/4062.chapter2.pdf
- https://www.researchgate.net/publication/316371245_Occurrence_and_Production_of_Aluminum
