Sulfur forms numerous allotropes, among them the two most important allotropes of sulfur are yellow rhombic sulfur (α-sulfur) and monoclinic (β-sulfur).
The sulfur content within the Earth’s crust is estimated to be approximately 0.6 percent by abundance. Abundant reserves of elemental sulfur can be found in sizable natural formations. In both Mexico and Sicily, the practice of mining and utilizing resources in their raw form, without the need for additional purification process processes, has been observed. One can find valuable sources of sulfur in the form of sulfur ores, such as:
Copper pyrites: CuFeS2
Iron pyrites: FeS2
Galena: PbS
Zinc blend: ZnS
Cinnabar: HgS
Sulfur, as an elemental entity, holds significant prominence due to its pivotal role in both industrial applications, particularly in the production of sulfuric acid, and its agricultural significance as a vital component of sulfate fertilizers. Sulfur holds significant importance within the realm of living systems as well.
Interesting Science Videos
Position of Sulfur in The Periodic Table
Sulfur, the second member of the oxygen family, is classified within group (VI) A on the periodic table. The ascending order of atomic numbers for the family’s members is as follows: Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), and Polonium (Po).
The electronic configuration of sulfur can be represented as 1s2 2s2 2p6 3s2 3p4. The general valence shell electronic configuration of this family is ns2 np4. Due to the fact that each member of the family has six valence electrons, they all belong to group (VI) A. Sulfur is comparable to other elements in the p-block. It is a non-metallic, solid substance. It occupies a spot within a second period of the periodic table, taking up a spot between the elements phosphorus and chlorine.
Allotropy of Sulfur
Sulfur, as an element, exhibits a remarkable propensity for the formation of various solid allotropes, surpassing all other elements in this regard. Currently, there exists a comprehensive understanding of approximately 30 distinct sulfur allotropes. The existence of multiple allotropes of sulfur can be attributed to the distinct arrangements of sulfur atoms within crystal structures. Due to the inherent dissimilarities in their crystal structures, distinct forms exhibit variations in density, external crystal morphology, coloration, thermal stability, and intrinsic energy. The following elucidations represent the various allotropic forms of sulfur:
Crystalline Form
(i) Rhombic or Octahedral or α-sulfur
(ii) Monoclinic or Prismatic or β-sulfur
Non Crystalline or Amorphous Form
(i) Plastic or γ-sulfur
(ii) Milk of sulfur or δ-sulfur
(iii) Colloidal sulfur
Rhombic or Octahedral Sulfur (α-sulfur)
Rhombic sulfur is the thermodynamically stable form of sulfur at ambient conditions. Among the various allotropes of sulfur, it can be observed that this particular one exhibits the highest degree of stability. This phenomenon implies that all alternative forms of sulfur eventually undergo a transition into α-sulfur. The common roll of sulfur or flowers of sulfur is classified within this particular category.
The natural color of elemental α-sulfur in its purest form is characterized by a greenish-yellow hue, whereas the commercially available variant exhibits a vibrant yellow color. The observed variation in color can be attributed to the inclusion of S7 at concentrations ranging from 0.1% to 0.5%, in addition to certain organic impurities. The occurrence of these impurities in the commercially available samples can be attributed to the production process of α-sulfur. The α-sulfur within its natural state is commonly found in association with minor quantities of less stable allotropes, predominantly S7.
Synthesis of Rhombic Sulfur (α-sulfur)
Pure α-sulfur can only be synthesized in a laboratory setting. When the crystals produced are octahedral, or eight-sided, it is possible to obtain them through the slow evaporation of a solution of roll sulfur in carbon disulfide (CS2). CS2 is a volatile liquid, and as such, it soon evaporates.
Properties of Rhombic Sulfur (α-sulfur)
- Rhombic sulfur exhibits transparency and possesses a pale-yellow hue.
- The α-sulfur exhibits solubility in various organic solvents such as carbon disulfide, benzene, and chloroform. However, it exhibits insolubility in aqueous solutions.
- The material exhibits poor thermal and electrical conductivity.
- The α-sulfur exhibits a melting point of 113°C, a boiling point of 445°C, and a specific gravity of 2.05.
- The substance, as indicated by its designation, exhibits crystallization in the octahedral rhombic structure.
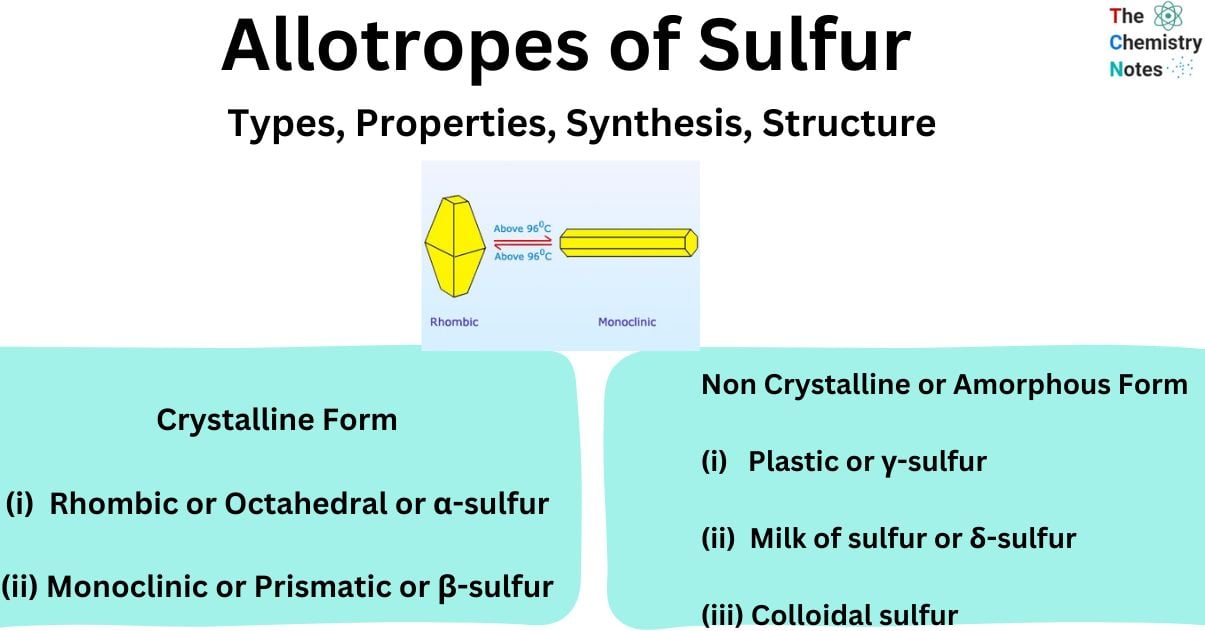
- Upon gradual heating to a temperature of 95.5°C, a transformation occurs, resulting in the formation of monoclinic sulfur.
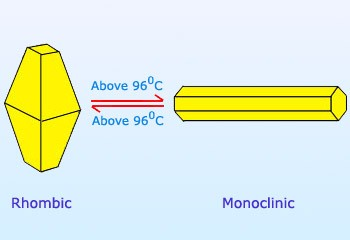
Structure of Rhombic Sulfur (α-sulfur)
Prismatic or Monoclinic Sulfur (β-sulfur)
The α-form of sulfur undergoes a phase transition to the β-form when exposed to temperatures exceeding 95.4°C. The β-form of sulfur is characterized by its monoclinic crystal structure and consists of S8 molecules. The meta-stable nature of this particular type of sulfur allows for the growth of sizable crystals through the process of cooling liquid sulfur. The β-sulfur undergoes a gradual transformation into the thermodynamically stable α-sulfur at temperatures lower than 95.4° C.
The crystals have the ability to be preserved for a duration of approximately one month prior to a notable portion undergoing transformation into α-sulfur.
Synthesis of Monoclinic Sulfur (β-sulfur)
The process involves the melting of elemental sulfur in a container, followed by controlled cooling until a solidified outer layer, or crust, is formed. A limited number of perforations are created in the outer layer, allowing for the drainage of the liquid. Upon the removal of the crust, needle-shaped crystals of monoclinic sulfur are obtained, exhibiting a long and prism-like structure.
Properties of Monoclinic Sulfur (β-sulfur)
- Monoclinic sulfur exhibits an opaqueness that is accompanied by a delicate pale-yellow hue.
- The β-sulfur exhibits solubility in carbon disulfide (CS2) while demonstrating insolubility in water.
- It is incapable of conducting heat and electricity.
- The β-sulfur exhibits a melting point of 119 °C and possesses a specific gravity of 1.96. The temperature within the range of 95.5°C to 119 °C typically exhibits remarkable stability.
- The crystals of monoclinic sulfur undergo a gradual transformation into the rhombic structure when they are stored at temperatures below 95.5°C, which is the established transition temperature for the interconversion of these two crystal forms. These substances are also referred to as enantiotropic substances. At a temperature of 95.5 °C, both forms remain stable and coexist.
Structure of Monoclinic Sulfur (β-sulfur)
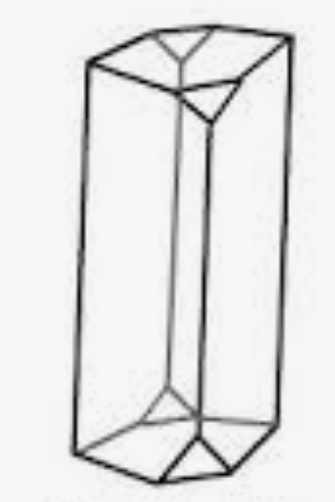
In both crystalline forms, the structural units consist of a single molecule that includes eight sulfur atoms (S8), which are linked together by covalent bonds within a puckered ring. Plastic sulfur does not consist of S8 rings but rather comprises elongated chains of sulfur atoms. These chains have the potential to be quite lengthy. Over the course of time, these chains go through a change, eventually returning to an S8 ring structure, resulting in the formation of rhombic sulfur.
Plastic or γ-sulfur
The γ-sulfur variant represents an additional monoclinic configuration of the S8 molecule. This particular compound represents a relatively steadfast manifestation of sulfur, which can be discerned within select mineral formations in the natural world. The compound in question may be synthesized through a meticulous process of gradual crystallization from a solution containing alcoholic polysulfides. The density of γ-sulfur, standing at 2.19 g/cm3, surpasses that of other prevalent allotropes of S8.
Synthesis of Plastic Sulfur (γ-sulfur)
When the process of introducing boiling sulfur into a body of cold water is undertaken, a remarkable transformation occurs, resulting in the formation of a solid substance possessing rubber-like properties, aptly referred to as plastic sulfur. The lack of crystallinity in this form arises from the rapid cooling of boiling γ-sulfur, which prevents the complete rearrangement of atoms into S8 rings. Consequently, the resulting plastic sulfur (γ-sulfur) is composed of both ring and chain molecules.
The elasticity of plastic sulfur bears resemblance to that of rubber owing to its inherent ability to undergo a reversal into a complex network of intertwined chains subsequent to being stretched. Upon subjecting plastic sulfur to the ambient conditions of room temperature, a fascinating phenomenon ensues. In a remarkable display of molecular reconfiguration, the constituent atoms undergo a process of collective rearrangement, ultimately resulting in the formation of ring-shaped molecules. This transformative event is accompanied by the loss of elasticity, a property previously exhibited by plastic sulfur. The plastic form of sulfur undergoes a transformation back into its crystalline, rhombic structure.
Properties of Plastic Sulfur (γ-sulfur)
- Plastic sulfur exhibits an opaqueness, presenting a dark reddish-brown hue, while simultaneously displaying an inherent instability.
- Plastic sulfur exhibits insolubility in both carbon disulfide and water.
- It possesses the inability to conduct both heat and electricity.
- Plastic sulfur, being in a super-cooled state of liquid, lacks a distinct melting point. Furthermore, its specific gravity is measured to be 1.95.
- Upon extended exposure to ambient temperature, the substance undergoes a transformation into a rhombic structure.
- Initially, the substance exhibits a pliable and resilient nature, yet subsequently undergoes a transformation into a solid state. The inherent elasticity of plastic sulfur arises from the phenomenon of chain unraveling in response to tension, followed by their subsequent recoiling upon the release of tension.
Milk of Sulfur (δ-sulfur)
Synthesis of δ-sulfur
Upon subjecting flowers of sulfur to the process of boiling alongside water and slaked lime, a chemical reaction occurs, resulting in the formation of calcium pentasulfide, denoted as CaS5. This compound can be obtained from the resulting solution, and upon the addition of concentrated hydrochloric acid, a white, shapeless variant referred to as milk of sulfur is precipitated.
3 Ca(OH)2 + 12 S → 2 CaS5 + CaS2O3 + 3 H20
(Calcium pentasulfide)
2 CaS5 + CaS2O3 + 6 HCl (conc.) → 3 CaCl2 + 3 H2O + 12 S
(milk of sulfur)
or, CaS5 + 2 HCl → CaCl2 + H2S + 4 S
Properties of Milk of Sulfur (δ-sulfur)
- The substance known as milk of sulfur is characterized by its opaque and white solid state.
- The compound known as milk of sulfur exhibits solubility in carbon disulfide (CS2), while it demonstrates insolubility in water.
- It lacks the ability to conduct both heat and electricity.
- The δ-sulfur possesses a specific gravity of 1.82.
- Over a prolonged period, the milk of sulfur possesses a tendency to undergo a reversion process, leading to the formation of the rhombic in variety.
Colloidal Sulfur
The production of colloidal sulfur usually requires the introduction of hydrogen sulfide (H2S) in water containing sulfur dioxide (SO2), or through the addition of sulphuric acid (H2SO4) to a saturated solution of sodium thiosulfate (Na2S2O3). The solution presents a certain degree of opacity due to the presence of sulfur, characterized by particle sizes ranging from approximately 10-5cm to 10-7cm.
Another approach entails combining a mixture of alcohol and sulfur with water. Furthermore, it functions as a solvent during the carbon disulfide reaction. Pharmaceutical applications can be facilitated through their utilization.
Applications of Sulfur
- In the context of rubber article production, the process of vulcanization is employed to produce vulcanized rubber. Vulcanization is a thermal treatment method involving the application of sulfur or its compounds to raw rubber, with the aim of eliminating its adhesive qualities and enhancing the longevity of rubber-based goods.
- This substance finds application in the fields of match industries and fireworks.
- Sulfur finds extensive application within the pharmaceutical industry.
- This substance is employed in the synthesis of H2SO4 as well as other compounds containing sulfur. Approximately 90 percent of sulfur is utilized in the production of sulfuric acid (H2SO4).
Frequently Asked Questions (FAQ)
What does octa-sulfur mean?
S8 is a molecule of sulfur also referred to as octa-sulfur. It is a solid that is brilliant yellow in color and is spongy and odorless. When this molecule is between its melting and boiling points, it polymerizes, increasing viscosity while decreasing density.
Which is the most stable allotrope of sulfur?
Sulfur mainly exists in two forms of allotropes: Rhombic and Monoclinic forms. Out of these, the rhombic allotrope (yellow in color) of sulfur is the most stable allotrope of sulfur.
What is the most common allotrope of Sulfur?
Rhombic Sulfur, also known as alpha(α) Sulfur, is the most common allotrope of Sulfur.
Are allotropes of Sulfur soluble in water?
Allotropes of Sulphur are not soluble in water.
Video on Formation of Allotrope of Sulfur
References
- https://wiki.aalto.fi/display/SSC/Allotropy+of+sulfur
- https://unacademy.com/content/jee/study-material/chemistry/allotropic-forms-of-sulphur-inorganic-chemistry/
- https://byjus.com/chemistry/sulphur-and-its-allotropic-forms/
- https://www.vedantu.com/chemistry/sulphur-and-its-allotropic-forms
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- Steudel, R., ed. (2004). Elemental sulfur and sulfur-rich compounds I (Topics in current chemistry). Springer. ISBN 3-540-40191-1
- https://www.geeksforgeeks.org/allotropes-of-sulphur/
- Housecroft, Catherine E.; Sharpe, Alan G. (2008). “Chapter 16: The group 16 elements”. Inorganic Chemistry, 3rd Edition. Pearson. p. 498. ISBN 978-0-13-175553-6.

