Phosphorus is highly reactive and therefore does not exist in its elemental form in nature. Phosphates tend to occur in an amalgamated state. The significant phosphate minerals include:
Phosphate rock or phosphorite: Ca3(PO4)2
Fluorapatite: 3Ca3(PO4)2.CaF2
Chlorapatite: 3Ca3(PO4)2.CaCl2
Vivianite: Fe3(PO4)2.8H2O
In addition to the above sources, phosphorus is also present in the connective tissue of plants as well as animals. The mineral calcium phosphate is a compound that is found in both teeth and bones. Bones typically consist of approximately 60-80 percent phosphorus content. Therefore, it is commonly referred to as the “bone element.” Phosphorus is indeed found in all living organisms in the form of organophosphates, such as adenosine triphosphate (ATP) and adenosine diphosphate (ADP).
| Element Name | Phosphorus |
| Symbol | P |
| Atomic Number | 15 |
| Electronic Configuration | [Ne] 3s2 3p3 |
| Density at Room Temperature | White: 1.823 g/cm3 Red: ≈2.2–2.34 g/cm3 Violet: 2.36 g/cm3 Black: 2.69 g/cm3 |
| Sublimation Point | Red: ≈689.2–863 K (≈416–590 °C, ≈780.8–1094 °F) Violet: 893 K (620 °C, 1148 °F) |
| Melting Point | White: 317.3 K (44.15 °C, 111.5 °F) Red: ∼860 K (∼590 °C, ∼1090 °F) |
Allotropy, also known as allotropism, refers to the scientific phenomenon wherein an element can exist in multiple distinct physical forms as well as structures. Allotropes are distinct forms of an element that exhibit different physical shapes. Allotropy is a phenomenon observed for particular chemical elements, wherein they possess the ability to be present in two or more distinct forms.
Different Allotropic Forms of Phosphorus
Phosphorus exhibits various allotropes, which are distinct forms of the element with distinct chemical and physical characteristics. Phosphorus exhibits several prominent allotropes, namely white phosphorus, red phosphorus, and black phosphorus. Furthermore, it is worth noting that violet phosphorous is also a known form of phosphorus. Black phosphorus is characterized by its black color, while white phosphorus exhibits a yellowish hue, and red phosphorus is distinguished by its red color.
In the natural environment, multiple allotropic forms of phosphorus can be found. The significant forms encompass:
(i) White or Yellow Phosphorus
(ii) Red Phosphorus
(iii) Black Phosphorus
(iv) Violet Phosphorus
(v) Scarlet Phosphorus
White or Yellow Phosphorus
White phosphorus, also known as tetra phosphorus and yellow phosphorus, is a chemical compound with various names. Natural extraction of white phosphorus is not feasible; however, it can be artificially produced through the simulation of processes involving phosphate rocks. White phosphorus is commonly known as a form of phosphorus that is obtained through either the retort process or the electro-thermal process. The white phosphorus possesses several distinct characteristics, which can be enumerated as follows:
Physical Properties of White or Yellow Phosphorus
- As a solid, pure phosphorus appears white. When exposed to sunlight, it turns from white to yellow, earning it the name “yellow phosphorus.”
- White phosphorus is a solid substance that exhibits a waxy appearance due to its translucent properties.
- The substance emits a distinct odor reminiscent of garlic.
- The substance exhibits corrosive properties and is highly toxic.
- White phosphorus is a malleable substance that possesses a relatively low hardness, allowing it to be readily cut using a knife.
- White phosphorus exhibits low solubility in water. The substance is typically stored in a water-based solution.
- White phosphorus is classified as a polar compound due to its solubility in carbon dioxide.
- The substance undergoes a phase transition from solid to liquid at a temperature of 44 °C and a phase transition from liquid to gas at a temperature of 287 °C.
- It possesses a specific gravity of 1.83.
Chemical Properties of White or Yellow Phosphorus
- Among the chemical characteristics of white phosphorus are:
- The substance undergoes a chemical reaction with atmospheric oxygen, resulting in combustion. Consequently, it is stored submerged in water as a safety precaution.
- It illuminates at night. The glow of phosphorus in the gloom is known as phosphorescence. The luminescence could be considered a cold flame.
- Its vapor density corresponds to the atomic structure of a tetra, but at high temperatures, it decomposes into P2.
P4 → 2P2
- When heated above 1700 °C, it becomes atomic phosphorus.
- When a metal reacts with white phosphorus, it forms a compound known as metal phosphide.
- White phosphorus undergoes an oxidation reaction upon exposure to moist air. This combination results in a luminous discharge characterized by sparkling. Consequently, it exhibits a conspicuous and uncontrolled emission of light.
Chemical Reactions of White Phosphorus
Action With Air
It is a highly active element whose combustion in air produces a yellowish-green flame that forms P2O3 and P2O5. This is referred to as phosphorescence. This flame is referred to as the cold flame because matchsticks do not ignite in it.
P4 + 3 O2 → 2 P2O3
P4 + 5 O2 → 2 P2O5
Action With Non-Metals
At normal temperatures, white phosphorus reacts with Cl2 gas to produce trichloride and pentachloride.
P4 (s) + 6 Cl2 (g) → 4 PCl3 (l)
P4 + 10 Cl2 → 4 PCl5
Similar reactions take place with Br2 and I2.
P4 (s) + 6 Br2 (g) → 4 PBr3 (l)
P4 (s) + 6 I2 (g) → 4 PI3 (g)
It produces phosphorus sulfide P2S3 or phosphorus pentasulfide P2S5 when combined with sulfur.
2 P + 3 S → P2S3
2 P + 5 S → P2S5
Action With Metals
Active metals such as Sodium (Na), Magnesium (Mg), and Calcium (Ca) undergo a chemical reaction with phosphorus upon heating, resulting in the formation of their corresponding phosphides.
3 Na + P [when heated] Δ → Na3P [sodium phosphide]
3 Ca + 2 P [when heated] Δ → Ca3P2 [calcium phosphide]
Action With Alkalies
White phosphorus reacts with strong alkalies to produce phosphine.
P4 + 3 NaOH + 3 H2O [when heated] Δ → 3 NaH2PO2 + PH3 (phosphine)
(sodium hypophosphite)
Synthesis of White Phosphorus
Phosphate rock undergoes industrial heating in the presence of carbon and silica within an electric furnace. Phosphoric acid is commonly employed as a means to extract the liberated phosphorus element in the form of vapor. White phosphorus has been employed as a weapon in various military contexts.
Structure of White Phosphorus
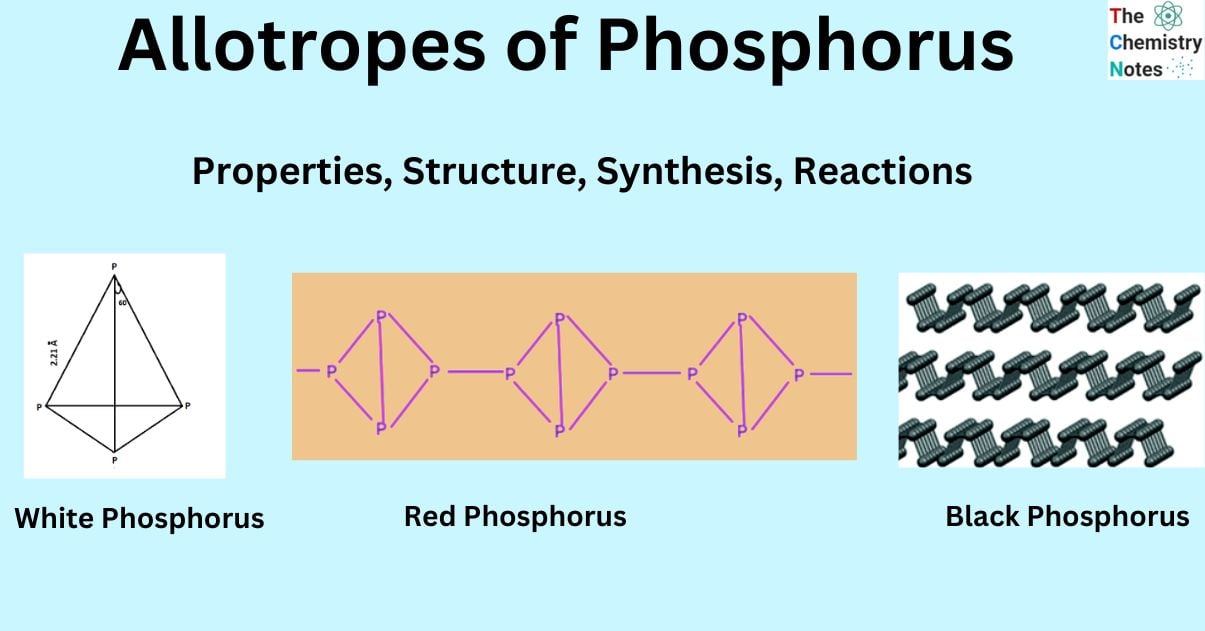
White phosphorus exhibits a molecular structure characterized by a ring-like configuration. The white phosphorus bond exhibits a bond angle of approximately 60 degrees. Three individual phosphorus atoms can form covalent bonds by sharing electrons. The particles exhibit mutual attraction due to the presence of weak Van Der Waals forces.
The white phosphorus molecule exists as a tetra atomic structure in both its vapor and solid states. The arrangement of four atoms is commonly observed at the corners of a tetrahedron.
Red Phosphorus
Red phosphorus is an alternative allotrope of phosphorus that can be obtained through the procedure that heats white phosphorus. The compound can be obtained by subjecting it to a high temperature of approximately 573 K (240-250°C) in the presence of an inert atmosphere for a prolonged period, along with a small amount of iodine. The reaction exhibits an exothermic nature, indicating a higher level of stability and lower reactivity compared to white phosphorus.
Red phosphorus possesses distinct characteristics and exhibits a well-defined arrangement, which will be explained in the parts that follow.
Physical Properties of Red Phosphorus
The following are some physical features of red phosphorus:
- Red Phosphorus has no taste or odor.
- It is a non-toxic violet-red substance.
- It has a melting point of 590°C.
- Red phosphorus does not glow in the dark.
- The molecular weight of red phosphorus is 30.97 g/mol.
- It has a specific gravity of 2.3.
- Red phosphorus exhibits insolubility in both water and carbon disulfide, while it demonstrates solubility in phosphorus tribromide (PBr3).
Chemical Properties of Red Phosphorus
The following are some chemical features of red phosphorus:
- Phosphorus exhibits a chemical reaction with oxygen at a temperature of 565 K, leading to the formation of phosphorus pentoxide.
- Additionally, it has been observed that this substance undergoes a chemical reaction with sulfur, leading to the formation of sulfides.
- Under typical conditions, this substance exhibits stability and does not undergo combustion in the surrounding atmosphere.
- Conversely, subjecting it to a temperature of approximately 400°C results in combustion.
- It does not decompose into white phosphorus that boils and resembles caustic soda. In essence, it decomposes into alcoholic potash.
Chemical Reactions of Red Phosphorus
White phosphorus exhibits higher reactivity compared to red phosphorus. The phosphorescence cannot be observed upon exposure to air. Under standard conditions of atmospheric pressure and temperature, phosphorus does not go through the combustion process. However, if exposed to an elevated temperature of 260°C, phosphorus undergoes combustion, resulting in the formation of phosphorus pentoxide (P2O5).
P4 + 5 O2 [when heated] Δ → 2 P2O5
Red phosphorus does not react with alkalis.
The transformation of red phosphorus to yellow phosphorus can be accomplished by subjecting it to elevated temperatures in an inert atmosphere, followed by the cooling of the resulting vapor.
Red P. [inert atmosphere 550-600°C] → Vapor [cooling] → White P.
When subjected to heat, it undergoes a chemical reaction with chlorine, resulting in the formation of phosphorus trichloride (PCl3) and phosphorus pentachloride (PCl5).
2 P + 3 Cl2 [when heated] Δ → 2 PCl3
2 P + 5 Cl2 [when heated] Δ → 2 PCl5
(Excess)
When subjected to the process of heating in the presence of sulfur, red phosphorus undergoes a chemical reaction that results in the formation of two compounds: di-phosphorus trisulfide (P2S3) and phosphorus pentasulfide (P2S5).
2 P + 3 S [when heated] Δ → P2S3
2 P + 5 S [when heated] Δ → P2S5
(Excess)
Synthesis of Red Phosphorus
White phosphorus undergoes a chemical transformation when subjected to a temperature of 573K in an environment devoid of reactive substances over an extended period. This transformation results in the formation of red phosphorus.
Structure of Red Phosphorus
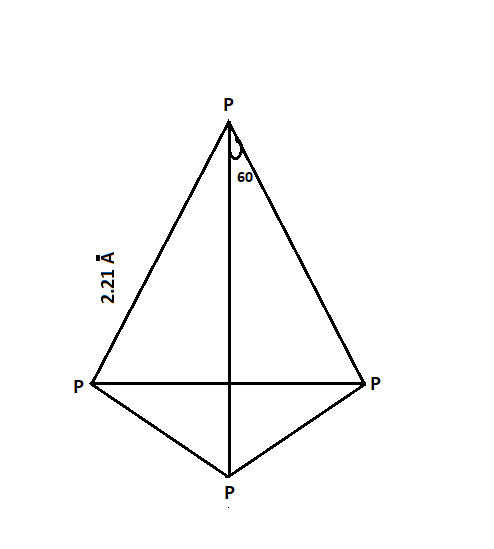
Red phosphorus possesses a similar atomic structure to that of black phosphorus. Red phosphorus is indeed a polymeric compound. The P4 molecule exhibits a tetrahedral molecular geometry, where each phosphorus atom is bonded to three other phosphorus atoms through covalent bonds. The reduced reactivity of white phosphorus can be attributed to its polymeric nature.
Black Phosphorus
The transformation of red phosphorus into black phosphorus happens via a method of heating at a temperature of 416°C. Black phosphorus can be obtained from red phosphorus through a technique involving heating the substance within a sealed tube at an appropriate temperature.
There exist two distinct variants of black phosphorus, namely α-black, and β-black. Upon subjecting red phosphorus to thermal treatment within a hermetically sealed tube at a temperature of 803 Kelvin, a distinct α-black variant is generated. The substance can undergo sublimation when exposed to air, resulting in the formation of rhombohedral crystals or opaque monoclinic structures. The substance exhibits no oxidation when exposed to atmospheric conditions. The transformation of the white form into β-Black occurs when it is subjected to extreme pressure as well as heat at a temperature of 473 K.
Physical Properties of Black Allotropes
Some of the physical properties of black phosphorus are included here:
- The object exhibits a black hue.
- The melting point of the black phosphorus is recorded to be 416°C.
- The specific gravity of the black phosphorus is 2.69.
- The molecular weight of the black phosphorus is 30.97 g/mol.
- The substance is present in both crystalline and amorphous states.
- The aqueous solubility of black phosphorus is reported to be 0.3 grams per liter (g/L).
Chemical Properties of Black Phosphorus
Black phosphorus is widely recognized as the most stable allotrope within the various allotropes of phosphorus.
It is the allotrope with the lowest reactivity.
Structure of Black Phosphorus
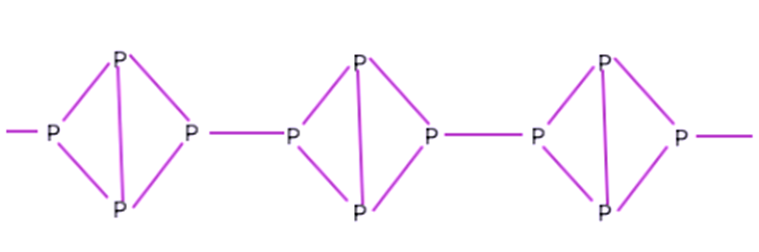
This phosphorus molecule possesses an arrangement that resembles a zigzag pattern made up of P-P bonds. Because of its primary resemblance to a honeycomb, this type of construction is referred to as a honeycomb structure. The bond angles add up to 99 degrees, and the bond length in this case is 218 pm.
Frequently Asked Questions (FAQ)
What kind of phosphorous is in a matchstick?
When a match is struck on a surface coated with red phosphorus, some of it is transformed into white phosphorus and ignites. The frictional heat causes the potassium chlorate to ignite, setting fire to the match head
Which allotropes of phosphorus are very toxic?
White phosphorus is the allotrope with the lowest stability, highest reactivity, highest volatility, lowest density, and highest toxicity. Light and heat speed up the process by which it transforms into red phosphorus.
Which is the most reactive allotrope of Phosphorus?
White phosphorus is the most reactive allotropes of phosphorus
Which is the thermodynamically most stable allotrope of phosphorus?
The lattice structure of black phosphorus is an interlinked ring of six P atoms. Here, each atom is bonded to three other atoms. This makes it a puckered sheet-like strongly interlinked structure, which is difficult to break. Hence, it is the most stable allotrope. This allotrope has the maximum amount of interlinking.
Why white phosphorus has the structure of P4 and sulfur can exist as S2?
White phosphorus has the structure of P4 as one phosphorus atom can form three bonds at a time. Thus, phosphorus forms a P4 white phosphorus tetrahedron (being sp3 hybridized), while sulfur can only form two bonds. Hence, sulfur only forms rings and chains.
References
- https://www.toppr.com/guides/chemistry/the-p-block-elements/phosphorus-allotropic-forms/#
- https://byjus.com/chemistry/phosphorous-allotropic-forms/
- https://www.vedantu.com/chemistry/allotropes-of-phosphorus
- https://infinitylearn.com/surge/chemistry/allotropes-of-phosphorus/
- https://www.embibe.com/exams/allotropes-of-phosphorus/
- Bridgman, P. W. (1914-07-01). “Two New Modifications of Phosphorus”. Journal of the American Chemical Society. 36 (7): 1344–1363. doi:10.1021/ja02184a002.
- Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 392. ISBN 978-0-13-039913-7.
- Durif, M.-T. Averbuch-Pouchot; A. (1996). Topics in phosphate chemistry. Singapore [u.a.]: World Scientific. p. 3. ISBN 978-981-02-2634-3.
