Alkanes are the simplest organic compounds made up of carbon and hydrogen only. They have the general formula CnH2n+2. They are also known as saturated hydrocarbons since their carbon skeleton is entirely saturated with hydrogens. Alkanes consist of strong C-C and C-H covalent bonds, so they are relatively chemically inert. They are also called paraffins. E.g. Methane, Ethane, Propane, etc.
Interesting Science Videos
Structure of Alkane
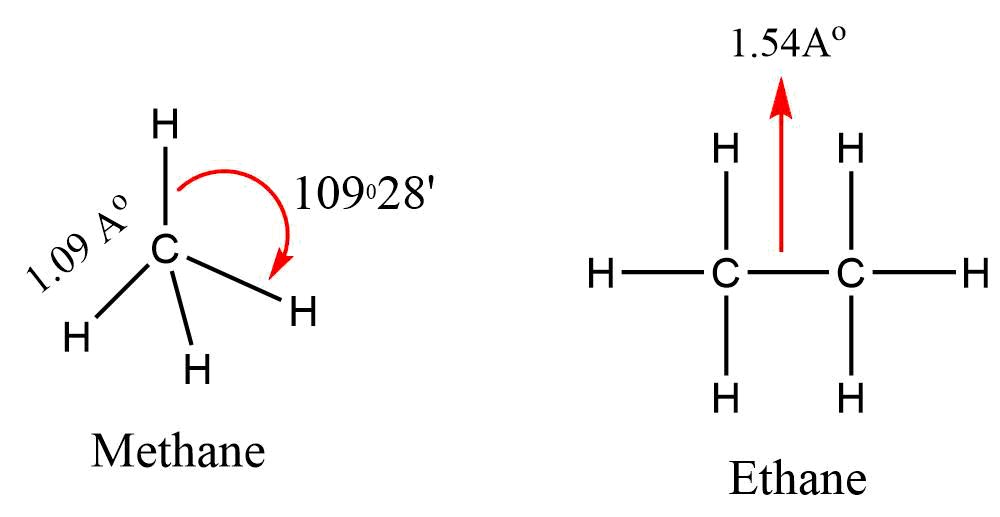
In methane, carbon forms four sigma bonds with hydrogen atoms. Carbon is covalently bonded with four hydrogen atoms. It uses its sp3 hybrid orbitals to form these bonds. While in the case of ethane there are six C-H covalent bonds and a C-C covalent bond. Each C-H bond is due to the overlap of the SP3 hybrid orbital from carbon and s orbital from hydrogen. The C-C bond arises from the overlap of the sp3 orbitals.
Alkanes Nomenclature
Common name system
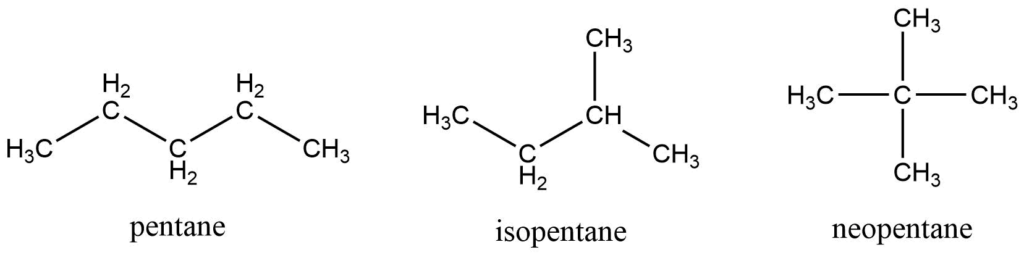
First, four members are called by their common names,i.e. methane, ethane, propane, butane. The names of bigger alkenes come from Greek prefixes that indicate the number of carbons in a molecule. For alkanes with a single continuous chain of carbons, the n-prefix is used. When a methyl group is linked to the second last carbon atom, the prefix iso is used, and when two methyl groups are attached to the second last carbon atom, the prefix neo is used.
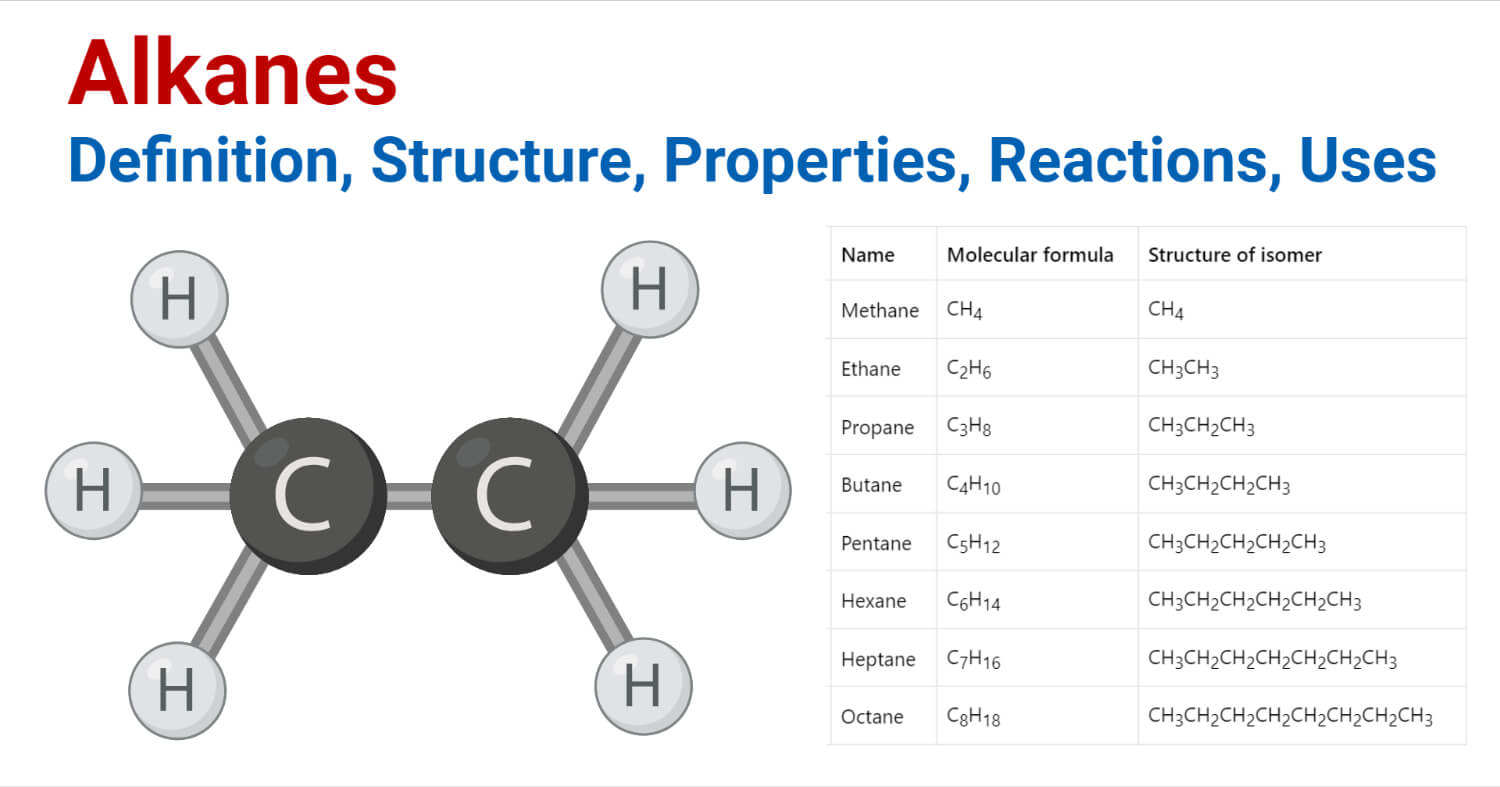
Name of some alkanes
| Number of Carbon atoms | Name | Molecular formula | Structure of isomer |
| 1 | Methane | CH4 | CH4 |
| 2 | Ethane | C2H6 | CH3CH3 |
| 3 | Propane | C3H8 | CH3CH2CH3 |
| 4 | Butane | C4H10 | CH3CH2CH2CH3 |
| 5 | Pentane | C5H12 | CH3CH2CH2CH2CH3 |
| 6 | Hexane | C6H14 | CH3CH2CH2CH2CH2CH3 |
| 7 | Heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 |
| 8 | Octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 |
IUPAC name systems
The naming of complex alkane isomers requires the use of a specific prefix. Since 1892, a committee and commissions of chemists have met regularly to assign a system of systematic nomenclature that could be utilized for difficult compounds. The IUPAC (International Union of Pure and Applied Chemistry) system was developed as a result of these discussions on the standardized naming of organic compounds.
Steps for the IUPAC naming system
Step 1: First, choose the longest continuous chain.
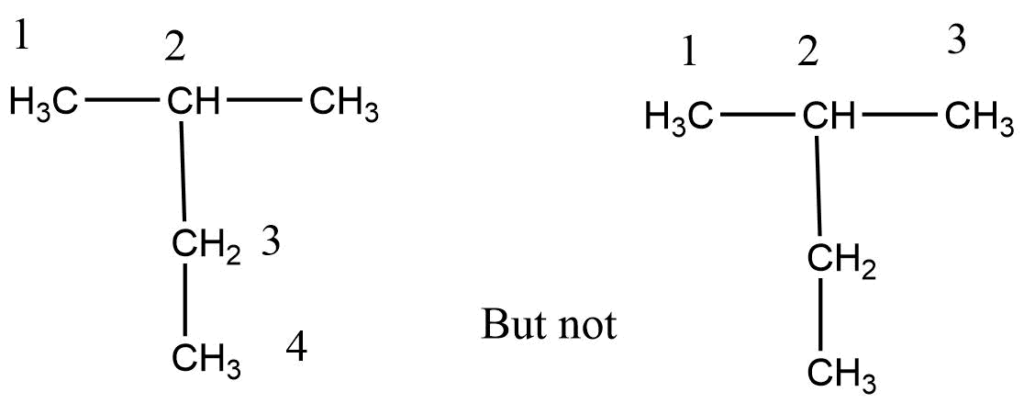
Step 2: The numbering process begins at that end, giving numbers with the lowest value to carbon-bearing substituents.
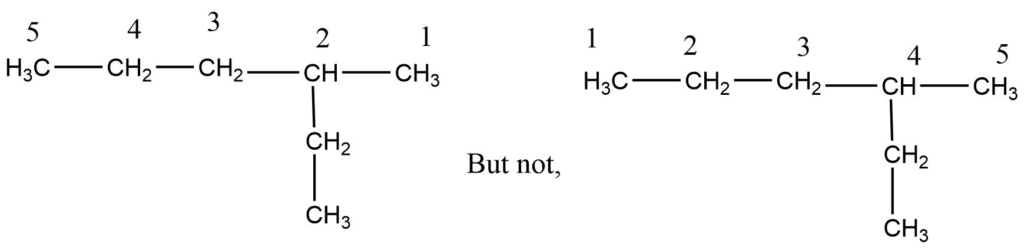
Step 3: Each substituent is named and positioned by the number of the carbon atom to which it is attached.
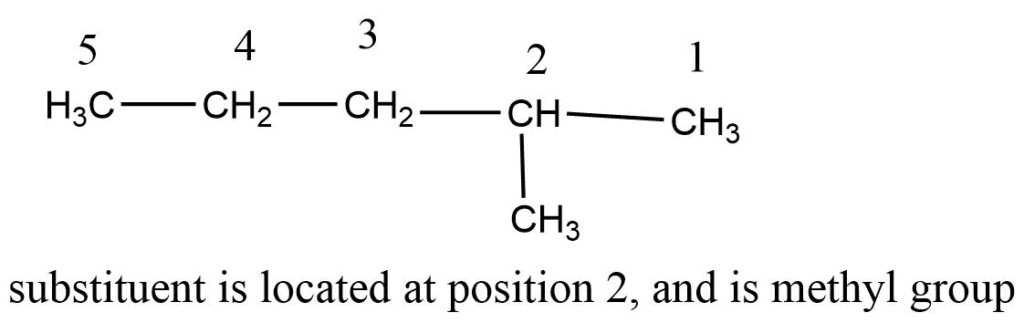
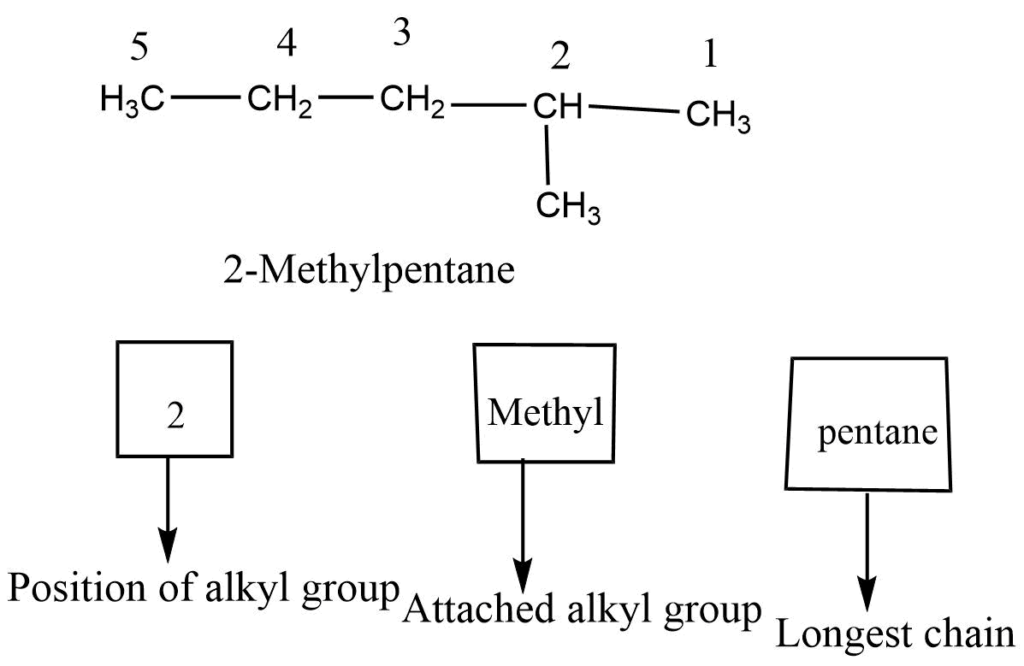
Step 4: Both the position and the name of the substituents are combined with the name of the longest chain to give the name in a single word.
Step 5: If two or more similar substituents are present the prefix di, tri, tetra, etc. is used to denote the number of substituents, their locations are indicated by different numbers separated by commas and placed directly before the name of the substituents.
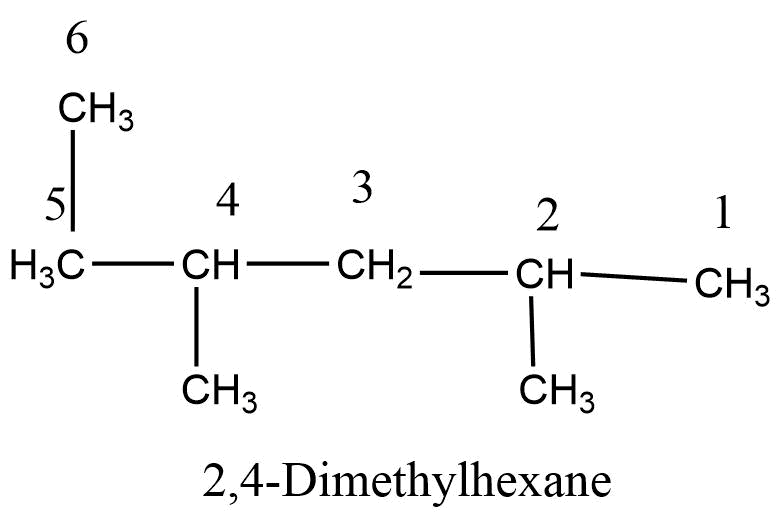
Step 6: If two or more substituents are used their name are alphabetized and added to the name of parent alkanes.
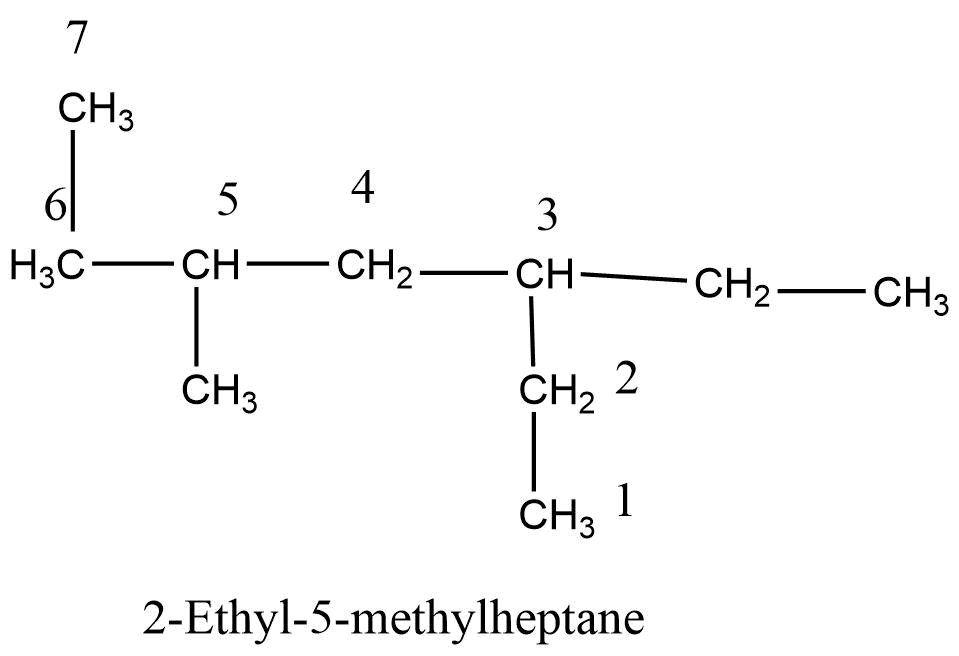
The structural formula of alkanes consists of four different types of carbon they are as follows:
- Primary carbon: Carbon attached to one other carbon is called primary carbon, i.e., 1° carbon.
- Secondary carbon: Carbon attached to two other carbon atoms is called secondary carbon, i.e., 2° carbon.
- Tertiary carbon: Carbon attached to three other carbon atoms is called tertiary carbon, i.e., 3° carbon.
- Quaternary carbon: Carbon attached to four other carbon atoms is called quaternary carbon, i.e., 4° carbon.
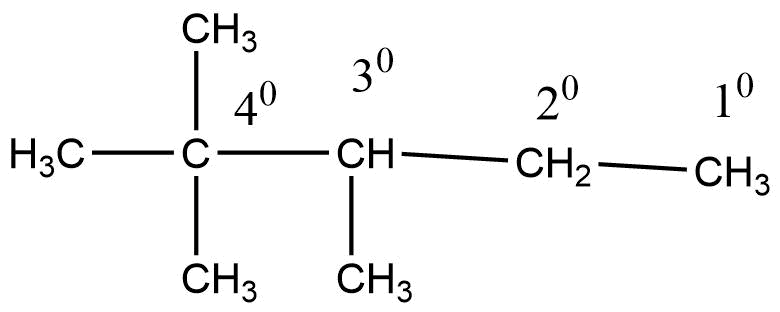
Hydrogens attached to 10, 20, and 30 carbons are called primary, secondary, and tertiary hydrogens.
Classifications of Alkanes
There are mainly three types of alkanes they are:
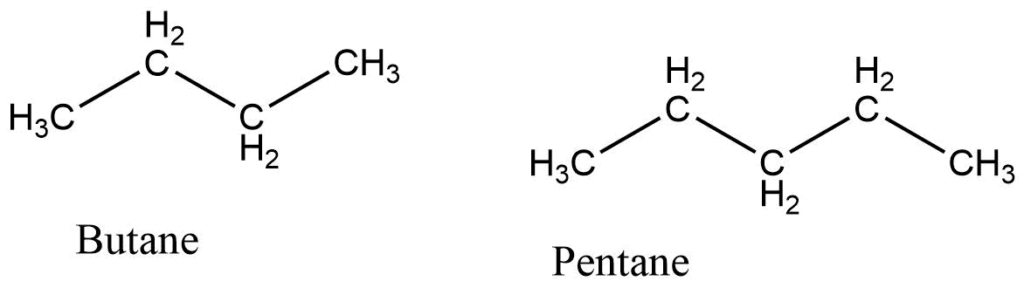
Straight chain alkanes
Alkanes made up of the straight hydrocarbon chain are called straight-chain alkanes. They are also called linear chain alkane. e.g.
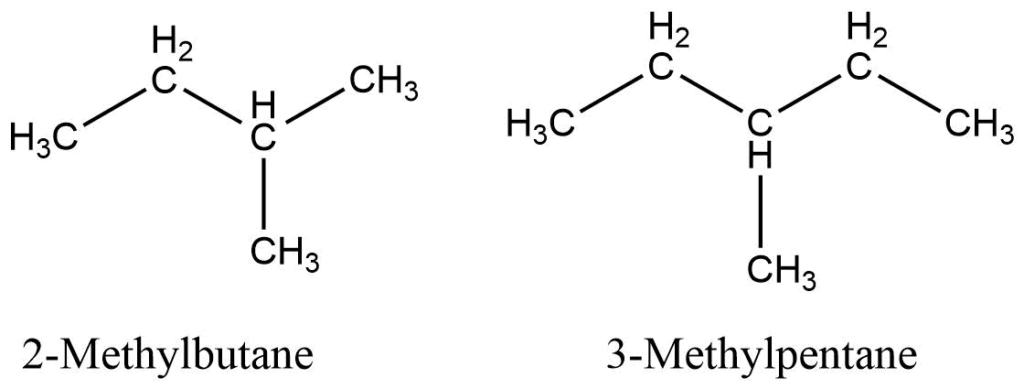
Branched-chain alkane
Branched-chain alkanes are alkanes with one or more alkyl branches on their straight chain. e.g.
Cyclic alkanes
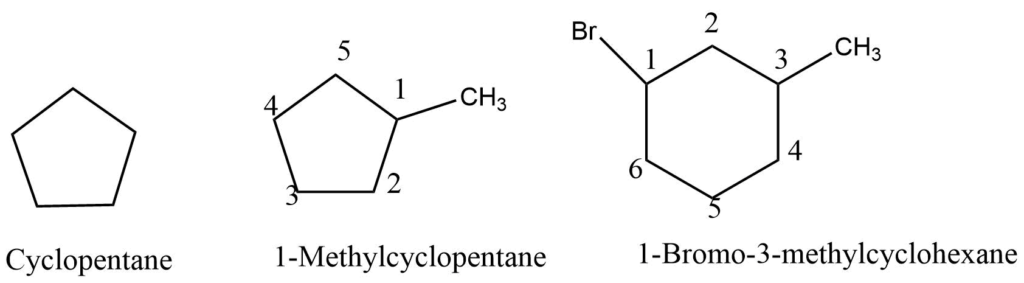
Alkanes in which the carbon atoms are linked to create a cyclic ring are commonly called cyclic alkanes. CnH2n is the general formula for cycloalkane. They are named by prefixing cyclo- to the number of carbon atoms in the name. The numbering of carbon begins with the importance of the functional group. The numbering will then proceed in the manner that offers the lowest number to the carbons, which contain a substituent group.
Sources of Alkanes
Natural gas and petroleum are the primary sources of alkanes. Both of these can be found in subsurface deposits together. The natural gas is composed of 80% methane, and 10% ethane and the remaining 10% is a mixture of higher alkanes. Petroleum is a major source of alkanes containing up to 40 carbons.
General methods of preparation of alkanes
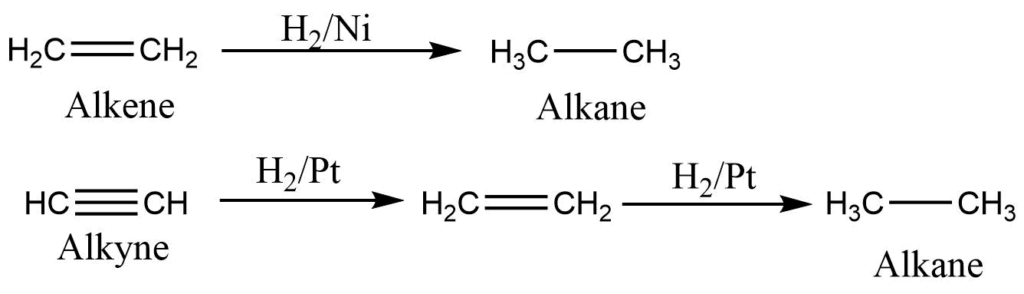
Hydrogenation of alkene or alkynes
Alkene and alkynes on reaction with hydrogen and nickel or platinum/palladium catalyst at 200-300oC forms alkane.
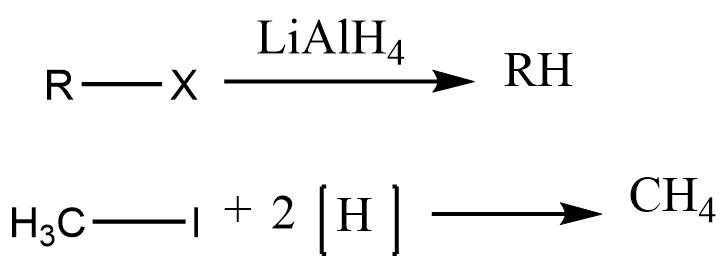
Reduction of alkyl halide
Alkyl halide in presence of reducing agents like LiAlH4, Zn+Hcl, Zn+CH3COOH undergo the reduction to form alkane.
Decarboxylation of carboxylic acid
Sodium salt of carboxylic acid when heated with soda lime (NaOH+CaO) alkane is formed. Thus, a produced alkane consists of one carbon less than the original acid.
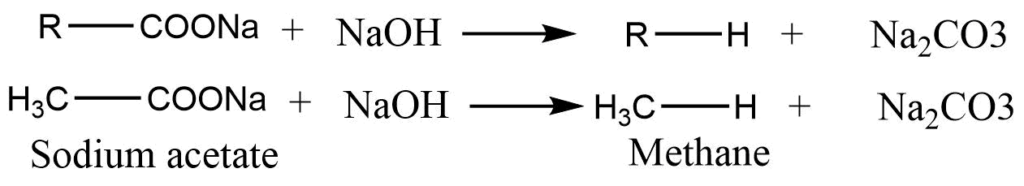
Hydrolysis of Grignard reagent
The Grignard reagent formed by the reaction of alkyl halide with magnesium in presence of dry ether, when treated with water give alkanes.
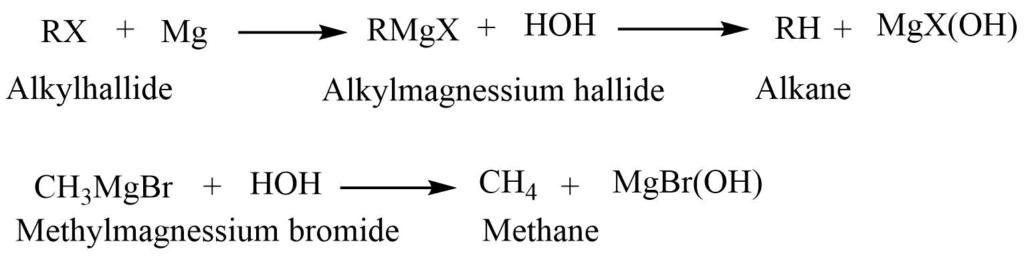
Wurtz synthesis
When two molecules of alkyl halide are heated with sodium metal in presence of ether alkane is formed. Two different types of alkyl halides give the mixture of alkanes. As a result, the difficulty in isolating the appropriate alkane product limits the utility of this reaction to the manufacture of symmetrical alkane only.
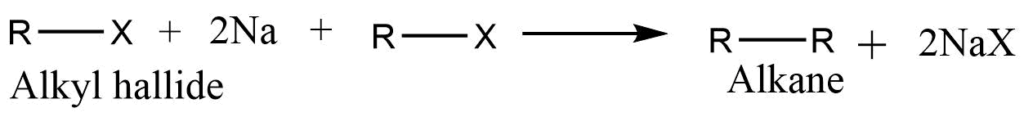
Laboratory preparation of alkanes
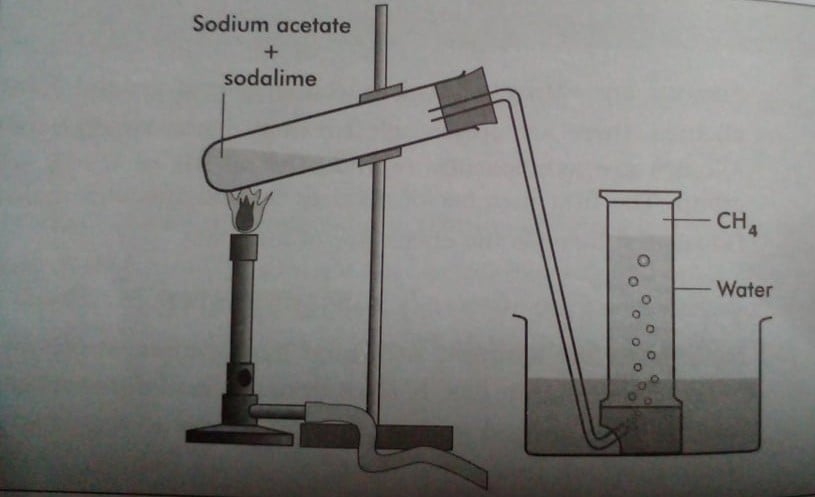
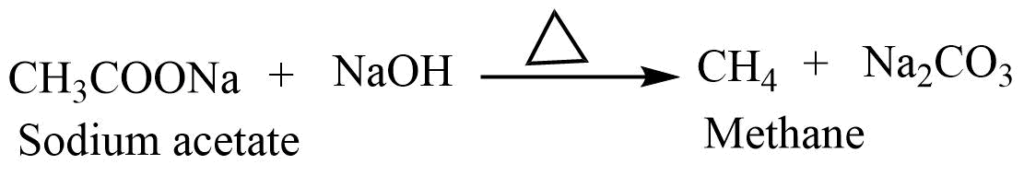
Laboratory preparation of methane from the sodium salt of acetic acid
In the laboratory, methane can be prepared by heating fused sodium acetate with soda lime in a 1:2 ratio. Soda-lime is less hygroscopic and doesn’t attack the glass-like sodium hydroxide so it is generally used for the preparation of methane.
Reaction involved:
Laboratory preparation of ethane from the sodium salt of acetic acid
When a concentrated solution of sodium acetate is electrolyzed between the platinum electrodes, ethane and CO2 are generated at the anode.
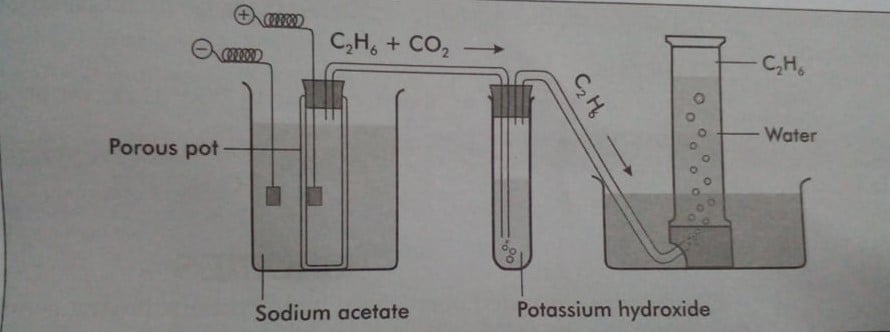
Reaction involved:
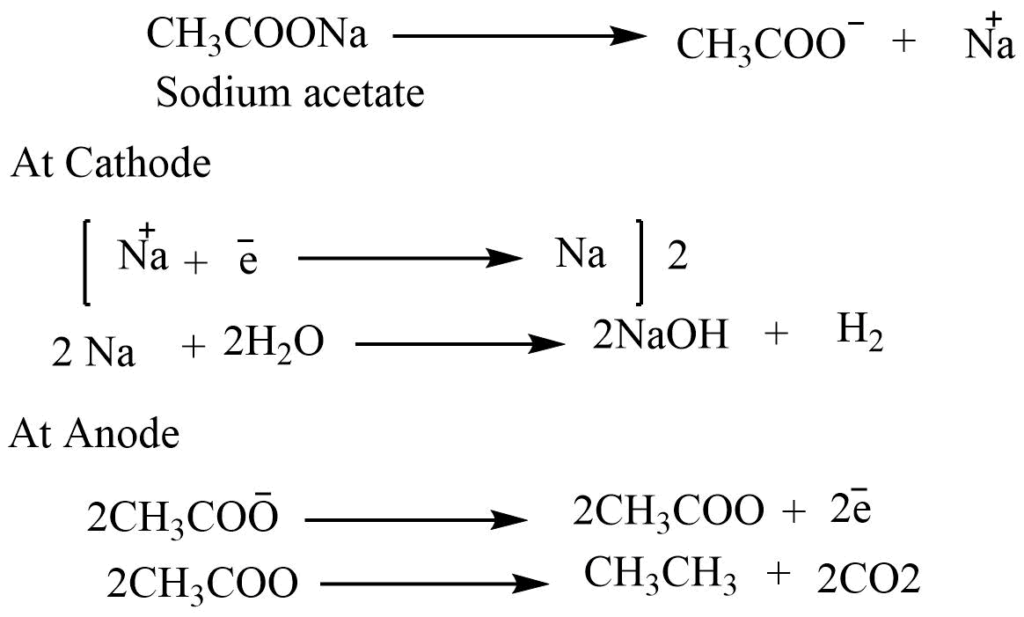
Properties of Alkanes
Physical properties of Alkanes
- Methane, ethane, propane, and butane are all present in the gas state. C5-C17 alkanes are colorless liquids, whereas higher alkanes are wax-like solids.
- Alkanes are nonpolar compounds. They are soluble in nonpolar solvents like benzene, carbon tetrachloride, etc.
- The boiling point of alkanes rises with increasing molecular weight. Straight-chain alkanes have greater boiling points than branched alkanes.
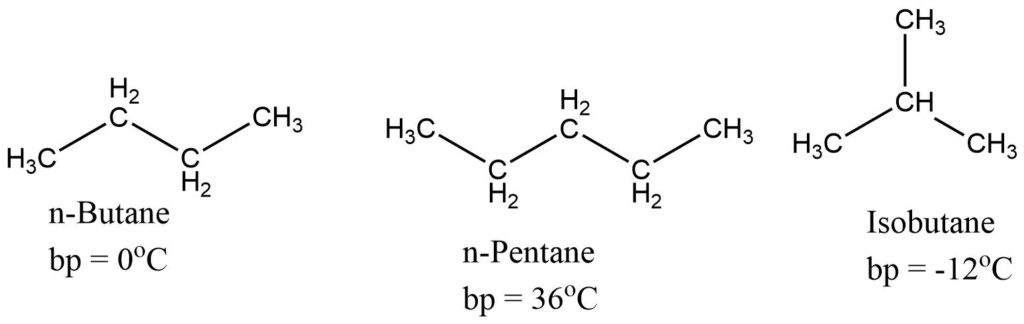
- There is no regularity in changing the melting point of alkanes. However, normally melting point increases with an increase in the molecular weight.
- IR spectrum of alkanes shows Characteristic C-H stretching frequency at 2850-3000 cm-1 and C-H bending vibrations at 1400-1470 cm-1.Chemical properties:
Chemical properties of Alkanes
At room temperature, alkanes are stable towards alkalis, acids, oxidizing, and reducing agents due to strong covalent sigma bonds between carbon-carbon and carbon-hydrogen. Some reactions of alkanes are as follows:
Halogenation reaction
In this reaction, the hydrogen atom of an alkane is replaced by a halogen atom
Chlorination reaction
Alkane on reaction with chlorine in the presence of sunlight at 300-400oC gives chloroalkane. The reaction proceeds continuously until chlorine atoms replace all hydrogen atoms.
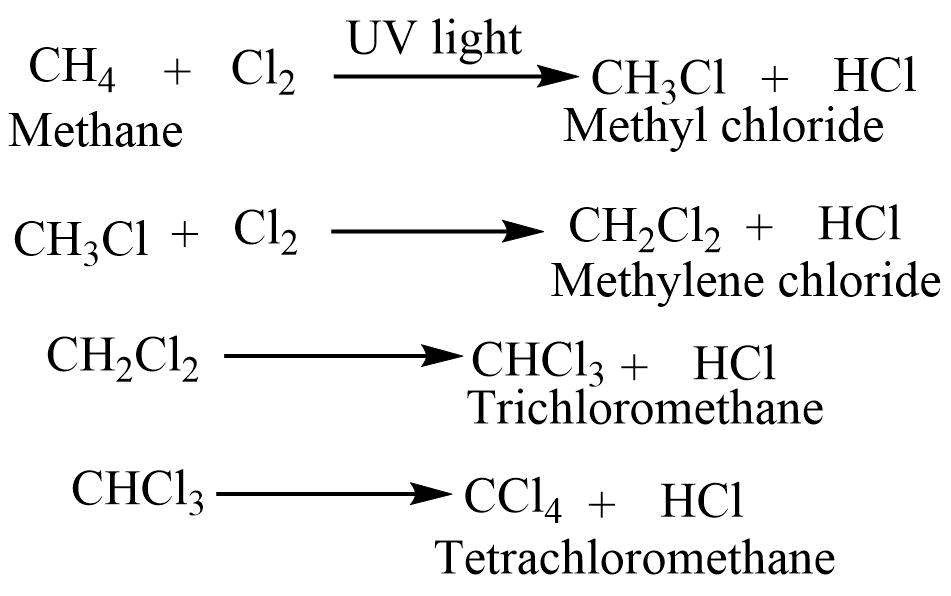
The product depends on the ratio of carbon and hydrogen if the carbon is present in an excess amount methyl chloride is the major one. When chlorine is present in an excess amount, tetrachloromethane is obtained.
Bromination reaction
Bromination reaction occurs in the same way as chlorination but less vigorously.
Fluorination
Flourine is most reactive. It reacts with alkanes explosively under most conditions.
Iodination reaction
The iodination reaction of an alkane is a reversible one as the hydrogen iodide formed during the reaction is a highly reducing agent which reduces the alkyl iodides to the alkanes. Iodination reaction in the presence of the oxidizing agent removes the hydrogen iodide and gives alkyl iodide.
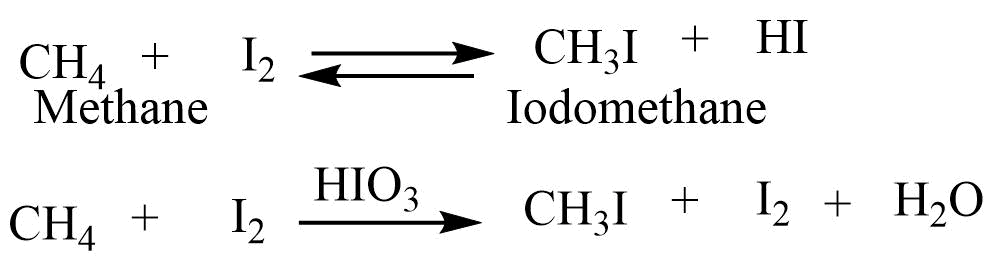
Nitration reaction
Alkane on heating with nitric acid vapors at 400-500°C gives the nitroalkane. The process is called the Vapour Phase Nitration.
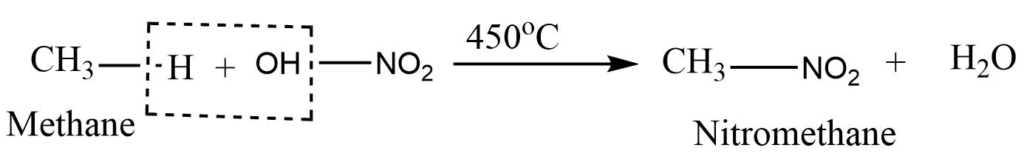
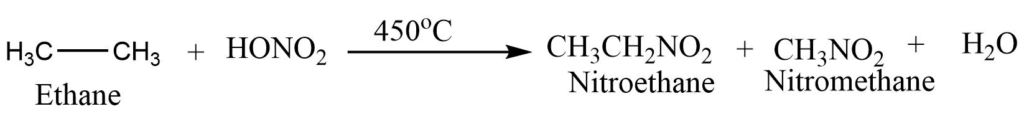
Sulphonation reaction
Alkane reacts with fuming sulphuric acid for a prolonged time to give alkane sulphonic acid. Lower alkanes like methane ethane do not give this reaction, Lower alkanes like methane ethane do not give this reaction.
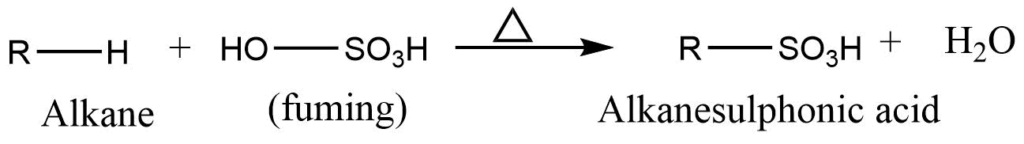
Combustion reaction
When an alkane is ignited in the presence of oxygen, it produces carbon dioxide and oxygen as well as a large amount of heat.
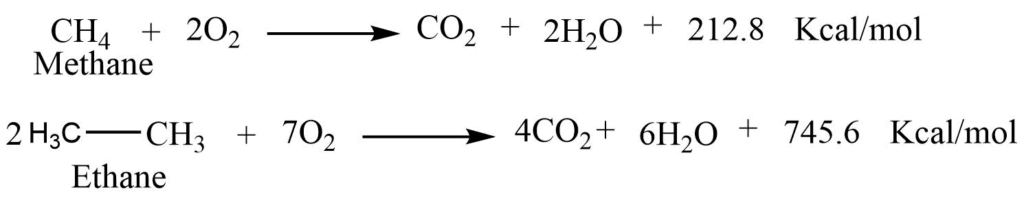
Pyrolysis or cracking
Larger alkanes are broken down into a mixture of smaller lower molecular weight alkanes, alkenes, and hydrogen when alkanes are burned to high temperatures in the absence of oxygen. This is called pyrolysis or cracking of alkanes. A temperature in the range of 500-800oC is generally used in this process. The reaction can occur at low temperatures in the presence of catalysts such as finely divided silica-alumina. This is known as catalytic cracking.
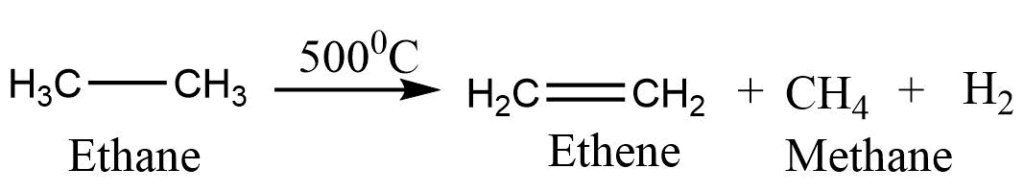
Uses of Alkanes
- Methane, ethane, propane, and butane are used as fuel gas.
- Semi-solid grease etc are used as Automotive and industrial lubricants.
- Vaselines are sued for making ointment and cosmetic products.
- Methane is used for the manufacture of methanol, methanal, and carbon black. Carbon black is used in printing inks and shoe polishes.
- Dehydrogenation of straight-chain alkanes in kerosene yields an alkene. When this reacts with benzene, it produces alkyl benzene, which is used in the production of detergents.
- Mineral oils having low boiling points are utilized as solvents for organic compounds.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- https://byjus.com/chemistry/alkanes/
- https://chemistrypage.in/why-methane-is-a-good-fuel-methane-gas/
- https://www.nextgurukul.in/questions-answers-forum/question/academic/kolbes-electrolysis/79518
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Hydrocarbons/Alkanes
- https://www.angelo.edu/faculty/kboudrea/molecule_gallery/01_alkanes/00_alkanes.htm
- https://www.bbc.co.uk/bitesize/guides/z3mpk7h/revision/3

I really appreciate the short and precise note
Thanks 👍😊 for the content you have provided
I love you
I appreciate you help
Thx
Thanks Soo much 👍👍👍
Thanks very much but more elaboration and explanation is needed
Acknowledgement,
but more elaboration and explanation is needed