In the context of actinide and lanthanide contraction, “contraction” refers to “the decrease in size” as the atomic number rises. As a result, actinide contraction describes the same phenomenon with chemical elements in the actinide series, but lanthanide contraction refers to the shrinkage in the size of an atom of a lanthanide with regard to the growing atomic number.
Interesting Science Videos
Actinide Contraction – Definition
Actinides gradually reduce their ionic radii as their atomic number rises, this process is known as actinide contraction. The nuclear charge and quantity of 5f electrons both rise by one unit in the actinide series.
The steady decrease in ionic radii with an increase in atomic number is referred to as actinide contraction. This actinoid contraction is caused because of the imperfect shielding by 5f-electrons.
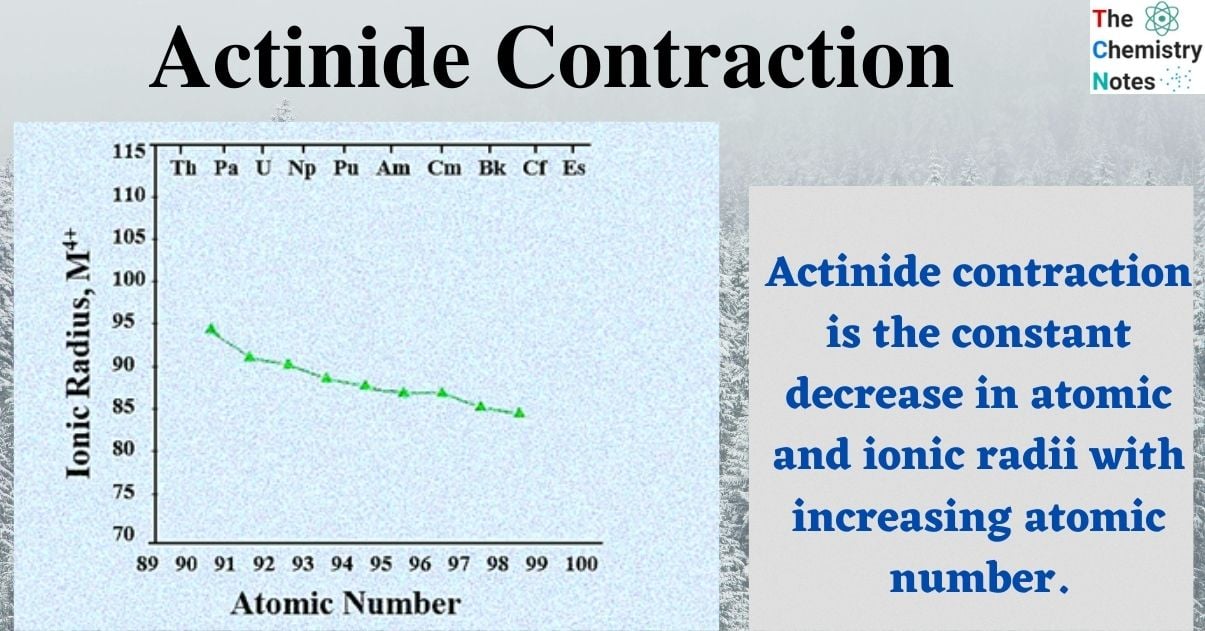
As we progress down the actinide series from thorium to lawrencium, the atomic and ionic radii of the actinide elements increasingly decrease. Thus, the ionic radius decreases from 109 pm to 98 pm after switching from Th 3+ to Lr 3+ ion. The An 4+ ion’s radii [An = actinide] are similarly steadily decreasing.
Actinide contraction, which is similar to lanthanide contraction, is the constant decrease in atomic and ionic radii with increasing atomic number of actinide elements, that is, positive nuclear charge.
Although the contraction in volume from one element to another is greater for actinide elements than lanthanide elements because of the 5f-inferior orbital’s shielding or screening effect compared to the 4f-orbital. F or 5f- contraction is another name for the actinide contraction.
Causes of actinide contraction
Actinide components typically have an electrical arrangement of 5f 1-14 6d 0-1 7s 2.
At each step along the actinide series from actinium to lawrencium, the differentiating electrons entered the inner 5f orbital by one.
The shielding or screening effect of the “5f”-orbital is now far smaller than that of the “s,” “p,” and even the “d”-orbital. This is a result of the “5f”-orbital’s form.
The poor shielding of the electrons in the 5f subshell is the main contributor to the Contraction. The size of the atom decreases as the atomic number rises from left to right due to the actinide contraction, in which the 5f electrons fail to protect both the outer shell electrons and other 5f electrons from the effective nuclear charge. The 4f electrons’ ineffective lack of protecting the outer shell electrons from the effective nuclear charge during the Lanthanide contraction results in a decrease in the atomic radius of the Lanthanide series.
It is stronger than lanthanide contraction because 5f shielding is weaker than 4f shielding.
Thus, each step results in an increase of one in the effective nuclear charge. The 5f electrons thus suffer a stronger inward pull. The size of the entire 5f n shell decreases as a result. The actinide contraction is determined by adding up all of the subsequent reductions.
Consequences of Actinoid Contraction
- Except for Ac, Th, Pa, and U, the majority of actinide elements are synthetic. The nuclear reaction creates them. Transuranic elements are the name given to these elements.
- The ionic size of the actinide elements is nearly the same. Because of this, it is challenging to separate actinide elements from one another. For instance, it is difficult to separate the elements Ac, Th, Pa, and U from uranium ores.
- Once more, this causes the ionic size along the actinide series to steadily shrink from Ac 3+ to Lr 3+. As a result, along the actinide series, the covalency of the compounds of actinide metal elements rises. In other words, actinium compounds are the least covalent and lawrencium compounds are the most covalent.
Difference between Lanthanide and Actinide Contraction
| Lanthanide contraction | Actinide contraction |
| Tri-positive lanthanide ions exhibit a steady decrease in atomic size or ionic radius from cerium to lutetium due to an increase in nuclear charge and the entry of electrons into the inner (n-2) f orbital. It is known as lanthanide contraction when there is a progressive reduction in size as the atomic number rises. | Due to increasing nuclear charge and electrons entering the inner (n-2) f orbital, the atomic size or ionic radii of tri positive actinides ions gradually decrease from Thorium to Lawrencium. Actinide contraction refers to this progressive size reduction as the atomic number increases. |
| Lanthanides have less size contraction. | Actinides have higher size contraction. |
| It involves the filling of the 4f orbital. | It involves the filling of the 5f orbital. |
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- https://kgghosh1990.medium.com/actinide-definition- causes-consequences-24a61ea2ae5
- https://dailyjustnow.com/en/what-is-actinoid-contraction- 59718/
